Figure 1.
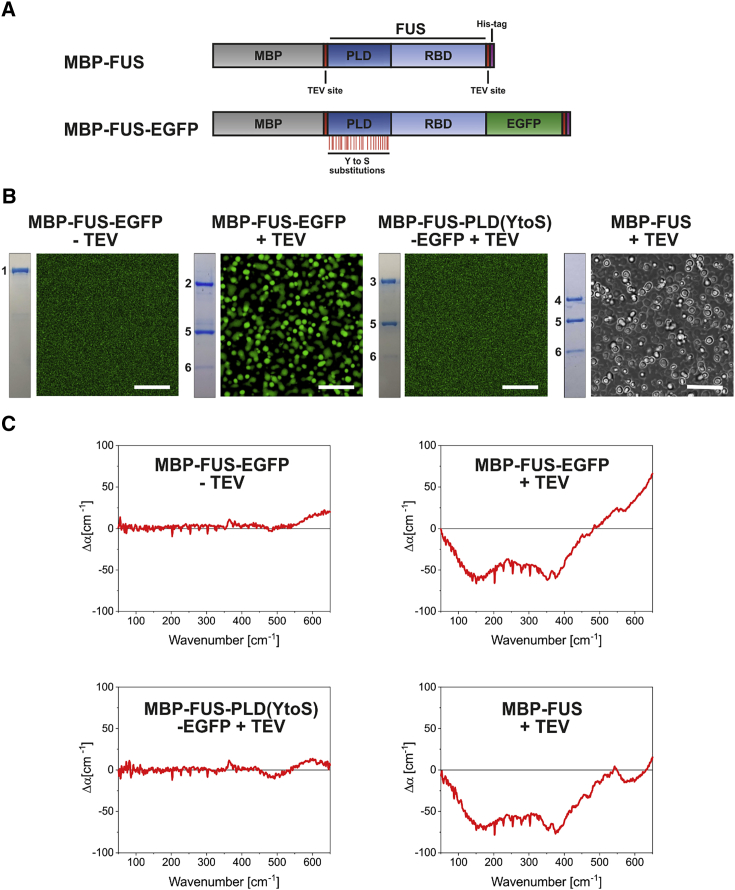
Terahertz fingerprint of LLPS of FUS. (A) Schematic drawing of the MBP-FUS and MBP-FUS-EGFP constructs is given. The constructs contain a His6 tag (violet) for purification and two TEV cleavage sites (red) to cleave off MBP and the His6 tag. FUS comprises the PLD (dark blue) and RBD domains (light blue). The Y to S substitutions are indicated as red lines below the PLD domain. (B) Microscopic images (50 × 50 μm; scale bar, 10 μm) of the protein samples (10 μM) are shown. The inset left of the image represents the corresponding sample analyzed by SDS-PAGE and Coomassie brilliant blue staining. Images of MBP-FUS were taken using brightfield illumination. (C) Averaged ATR-FTIR Δα spectra (50–650 cm−1) of protein samples measured 60 min after deposition on the ATR crystal surface are shown. On the left side, we show Δα spectra (50–650 cm−1) of samples that do not undergo LLPS, and on the right-hand side, spectra of samples after LLPS. The average error of all ATR-FTIR measurements is 5 cm−1. To see this figure in color, go online.