Abstract
Phase separation of biological molecules, such as nucleic acids and proteins, has garnered widespread attention across many fields in recent years. For instance, liquid-liquid phase separation has been implicated not only in membraneless intracellular organization but also in many biochemical processes, including transcription, translation, and cellular signaling. Here, we present a historical background of biological phase separation and survey current work on nuclear organization and its connection to DNA phase separation from the perspective of DNA sequence, structure, and genomic context.
Phase separation of biological molecules, such as nucleic acids and proteins, has garnered widespread attention across many fields in recent years. For instance, liquid-liquid phase separation has been implicated not only in membraneless intracellular organization but also in many biochemical processes, including transcription, translation, and cellular signaling. Here, we present a historical background of biological phase separation and survey current work on nuclear organization and its connection to DNA phase separation from the perspective of DNA sequence, structure, and genomic context.
Introduction
Compartmentalization of the cellular interior is an important part of life processes (1). This past decade has seen resurgence of interest in membraneless compartmentalization achieved via phase separation of proteins and nucleic acids (2, 3, 4, 5). Many of these studies implicate liquid-liquid phase separation (LLPS) as the physical principle behind the formation of membraneless compartments inside cells (3). The term LLPS in this context refers to segregation of one or more macromolecules into a concentrated liquid phase and a dilute phase depleted of the macromolecules. LLPS of biological macromolecules have also been referred to as liquid phase condensation, and the resulting concentrated phases have been termed biomolecular condensates (4,5).
Several proteins, typically highly charged and/or enriched in disordered regions, have been shown to readily undergo LLPS in vitro (6). Databases now exist dedicated to compiling proteins that are either predicted or have been experimentally shown to undergo LLPS (7, 8, 9, 10). The phase behavior of a protein depends on external environmental factors, including temperature (11,12), ionic strength (13,14), or pH (15,16). Furthermore, the phase transitions are often seeded/tuned by the presence of RNA (17,18), DNA (14,19), or free nucleotides (19,20). Nucleic acids can have a complex effect because both physical (structural/mechanical) and chemical properties of DNA can influence the phase transitions (14,21, 22, 23, 24). For instance, DNA hybridization and local sequence-dependent flexibility selectively promote either precipitation or LLPS (14,21). Chemical modifications through nucleotide methylation have also been shown to affect DNA phase separation (23,24). Additionally, when considering DNA-dependent phase separation within the nucleus, several features of the genomic DNA, such as the distribution patterns of DNA sequence elements (CpG islands (25), repeat elements (26), and specific protein recognition elements (27,28)), the number and affinity of protein-binding DNA motifs (29), and sequence features of the DNA that can affect nucleosome positioning (30,31), can contribute to the formation and stability of phase-separated condensates.
Much of the current physical understanding of LLPS (32,33) is based on the theoretical framework of phase separation of polymers. Prediction of the phase diagram, temperature dependence and critical temperature, and effect of intermolecular interactions on phase separation is based on modified Flory-Huggins theory, which describes the thermodynamics of phase separation of uncharged polymers. The theory expresses the free energy of a polymer solution as the sum of the mixing entropy and the polymer-polymer, polymer-solvent, and solvent-solvent interactions (34). To describe the phase behavior of charged polymers (polyelectrolytes), Voorn and Overbeek modified Flory-Huggins theory by adding a Debye-Hückel term to the free energy expression to describe electrostatic interactions (35). Although the Voorn-Overbeek theory was successful at modeling simple polyelectrolytes (36,37), the shortcomings become apparent when attempting to describe biological phase separation, which involves polyelectrolytes with nonuniform charge distribution and multivalent interactions. Modern theories aimed at describing biological phase separation attempt to account for complex charge distributions and charge correlations along the polymer backbone (33,38,39). One such approach, termed spacers-and-stickers model, coarse grains a protein into “sticker” segments with associative interactions separated by inert “spacer” segments (33,40,41). The system is then characterized by the strength and type of associative interactions and the average spacing between sticker segments, which can be estimated from the protein structure. This approach has been successful in reproducing experimentally measured phase diagram and temperature dependence of LLPS of prion-like domains (41).
LLPS has been implicated in many biological functions, including spatial organization of chromatin (42, 43, 44), transcription (45,46), translation (47), DNA damage repair (48,49), cellular signaling (50,51), and immune response (52,53). Despite the apparent success of LLPS as a general description of biological phase separation, the field has not yet obtained a unified view on what in vivo observables are indeed characteristic of LLPS. Recent debates and discussions on the methods determining phase separation in vivo can be found in the following reviews (54, 55, 56). Alternative mechanisms for protein localization in vivo have also been put forward, including polymer-polymer phase separation (PPPS) (57) as well as transient multivalent interactions without LLPS (58). The importance of identifying the correct mechanism goes beyond semantics because many biophysical and biochemical processes occurring within biological condensates depend on the underlying physical mechanism (57). Key differences between these mechanisms include the dependence on protein concentration, the regulation of the condensate size and concentration, and the transport properties within the condensates. LLPS requires a critical protein concentration to drive phase separation, and once formed, the concentration within the phase-separated droplet is regulated by the chemical potential of the concentrated and dilute phase. In addition, partitioning/exclusion of “spectator” species (small molecules or proteins that do not directly contribute to phase separation) is determined by the chemical properties of the molecule or the protein. In contrast, PPPS and transient multivalent interactions predict that the intermolecular interactions can localize and concentrate proteins even at low protein concentrations. The concentration within the condensates is determined by the number of binding and interaction sites (57,58). Furthermore, partitioning and transport of spectator species within the condensates are based on size exclusion not chemical properties (57). Therefore, characteristics of the condensates, including size of nucleation sites, condensate size and concentration, and partitioning and transport properties, depend on the physical mechanism.
In this review, we focus mainly on LLPS of DNA in a biological context, beginning with a historical perspective of nucleic acid phase separation and early descriptions of liquid-like compartmentalization within the nucleus. We then discuss the potential relationship between DNA sequence features and genomic context in phase separation of membraneless nuclear compartments and domains. Lastly, we discuss an emerging theme regarding the role of DNA mechanical properties, determined by the hybridization, sequence, and chemical modifications, on the phase behavior.
History of the biological LLPS concept
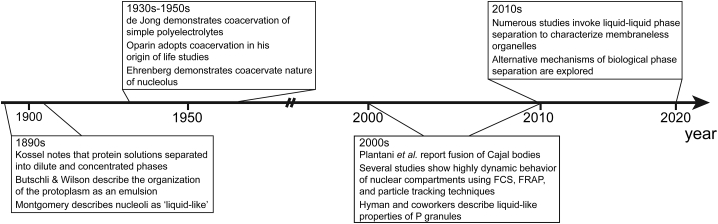
The discovery of phase separation of proteins and nucleic acids can perhaps be credited to Albert Kossel, who noted that solutions of proteins can separate into layers: a depleted phase and an enriched phase (59, 60, 61). Although we are only beginning to unravel the multidimensional functional consequences of phase separation, the concept of phase separation as the organizing principle of the cellular interior has existed since the late 1800s (Fig. 1). Otto Bütschli (62,63) depicted the protoplasm (protoplasm is an obsolete term that was used to refer to the cellular interior as a basis of life) as “alveolar” (emulsion-like). In his published lecture, E. B. Wilson, drawing on his light microscopy experiments with the naturally pigmented Ophiura eggs, described “liquid-droplet”-based organization of the protoplasm (64) consistent with Bütschli’s depiction. Additionally, the physical property of one of the earliest known membraneless organelles—the nucleolus—was also described in the late 1800s as liquid-like, as inferred from observations of fusion and growth of smaller nucleoli (using nucleolar stains) under a light microscope (65).
Figure 1.
Abbreviated timeline of studies of phase separation in biology. The discovery that charged proteins can spontaneously segregate into concentrated and dilute phases that date back to the 1890s. Around the same time, initial descriptions of the cell interior invoked the formation of liquid-like emulsions as the organization principle of the cell. Decades later, systematic studies of phase separation of polyelectrolytes, termed complex coacervation, provided a general framework to understand the membraneless organelles like the nucleolus. In the 2000s, significant progress was made, revealing the dynamic nature of several nuclear compartments and the rapid exchange of their components. Subsequent work explained the formation of P granules by LLPS. This work helped draw significant attention of the biological community toward LLPS, which has been considered as the mechanism underlying the formation of a number of membraneless compartments in cells.
Although “LLPS” has recently become the more commonly used term, it is analogous to “complex coacervation.” This phenomenon is observed when two or more macromolecules with associative interactions, such as electrostatic interactions in oppositely charged polyelectrolytes, are mixed in aqueous solutions under appropriate conditions (66). Descriptions of coacervation arose in the 1930s from extensive studies by Bungenberg de Jong (67). He used the term “coacervate” to refer to the layer (or drops as observed under a light microscope) enriched in dissolved substances that separated from the dilute (depleted in dissolved substances) phase. “Biomolecular condensate” is a newer term used to refer to highly concentrated droplets, gels, or reversible aggregates formed via associative interactions of biological macromolecules, and therefore, “complex coacervates” can be considered a subset of biomolecular condensates.
Bungenberg de Jong studied a variety of mixtures of hydrophilic biological polymers and discussed potential implications of coacervation in biology, including the formation of the nucleolus (67). Based on the realization that the nucleolus consists of high concentrations of histone-like (positively charged) proteins and ribose nucleotides (negatively charged), Frey-Wyssling hypothesized that the nucleolus is a coacervate (67,68). This was supported by temperature-dependent experiments on the nucleoli of fixed and stained root meristems by Lars Ehrenberg in the 1940s (69). The phenomenon of coacervation was also adopted by Alexander Oparin during his “Origin of Life” studies in which he proposed that the enrichment of concentration resulting from complex coacervation was the mechanism of formation of primordial cells (70).
In the late 1900s, in vitro studies (using methods such as optical and electron microscopy, birefringence, rheology, and turbidity measurements) of phase separation of DNA in contexts such as DNA condensation, liquid crystal formation, and gene delivery were quite popular (71, 72, 73). Several studies have shown that DNA forms liquid crystals at high solution concentrations in the presence of polymers that induce osmotic stress as well as multivalent polycations and polymers (74). Leforestier and Livolant proposed the chromatin to have local liquid crystalline domains (71). In dinoflagellates, which are single-celled eukaryotes with large genomes, liquid crystalline DNA have been observed using polarized light microscopy and electron microscopy (75).
After Finch and Klug’s proposal of the solenoid model of chromatin in 1976 based on electron microscopy images (76), the chromatin was largely assumed to be organized into 30-nm chromatin fibers (77). This model, however, has been heavily debated (78,79). Several studies suggest the chromatin to be more disordered and liquid-like (80,81). More recently, the functional role of LLPS at the genetic level has garnered attention with studies, implicating LLPS in the segregation of genes into transcriptionally active and repressive regions (42, 43, 44). This is consistent with liquid-like descriptions of interphase chromatin as well as observations of highly dynamic exchange of chromatin-associated proteins observed in many early in vivo experiments as discussed below (82, 83, 84, 85).
Besides the chromatin itself, liquid-like properties of several nuclear bodies were reported in many studies. For example, Platani et al. (86) in 2000 reported fusion events of Cajal bodies, which are nuclear compartments involved in biogenesis of small ribonucleoproteins. Before LLPS was a widely observed phenomena in biology, studies had demonstrated high mobility of proteins inside cellular bodies, such as promyelocytic leukemia protein (PML) bodies (87), nuclear speckles (88), and Polycomb bodies (89), using in vivo fluorescence correlation spectroscopy (FCS), fluorescence recovery after photobleaching (FRAP), and/or particle tracking techniques for nuclear compartments. The observation of high mobility proteins established the dynamic nature of the bodies, which allows exchange of the proteins with the surroundings. High mobility of proteins, although not defining, is a characteristic feature of membraneless organelles formed via LLPS.
As observations of proteins undergoing phase separation have become increasingly common, renewed critiques have emerged regarding whether observations of dynamically localized proteins in vivo are correctly attributed to LLPS. In concert, alternative mechanisms for the formation of membraneless bodies have also been proposed. In 1995, Walter and Brooks hypothesized that compartmentalization of the cytoplasm occurs via phase separation arising from macromolecular crowding (90). In 2007, Iborra suggested that differences in dynamic mobility of nuclear components could be a driving force in compartmentalization (91), similar to viscoelastic phase separation previously described in simple polymer solutions (92). Interpretations of chromosome conformation capture data, such as Hi-C contact maps, have invoked block copolymer phase separation in chromatin compartmentalization (93,94), in which weak attractions between similar sections of A-B-structured polymers drive segregation (95). Alternatively, PPPS can drive phase separation and compaction via bridging proteins that can bind to multiple segments of a polymer (57). This mechanism has been argued as the underlying mechanism of heterochromatin organization by HP1 owing to the bridging nature of HP1 (96). Recently, using live-cell, single-molecule imaging, Tjian and co-workers studied the formation of local compartments enriched in low-complexity domains of transcription factors (“high concentration hubs”) (58). They showed that at endogenous levels of the transcription factors, selective, dynamic interactions between the low-complexity domains drive their assembly into such compartments via transient multivalent interactions with no detectable LLPS. In light of emerging alternative mechanisms for membraneless compartmentalization, it is clear that more rigorous experimental methods for differentiating mechanisms of phase separation in the nucleus are needed.
Phase separation and compartmentalization in the nucleus: role of DNA
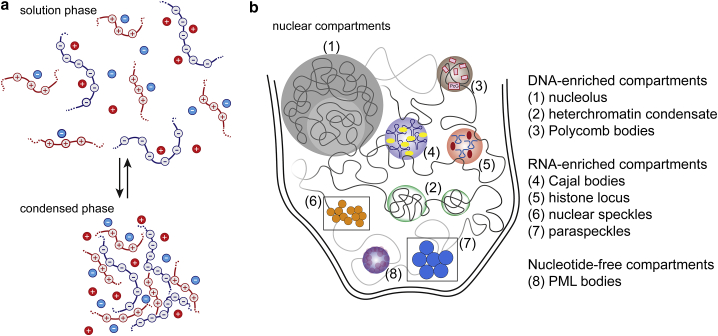
The genomic DNA in eukaryotes is hierarchically organized in the nucleus. The first level is in the form of the nucleosome core particle that packs ∼140-bp-long DNA with the help of core histones (97). Linker histones then package the nucleosome core particles into chromatin (98). Chromatin fibers appear randomly interwoven during the interphase of the cell cycle, though the spatial distribution is known to be highly organized (99). Each chromosome is known to occupy a distinct region in the nucleus, referred to as chromosome territory (100,101). Originally defined based on staining with simple dyes, chromatin is segregated into densely staining heterochromatin and lightly staining euchromatin (102), which occur on the length scale of ∼100 kbp to ∼1 Mbp DNA. Heterochromatin, further categorized as constitutive and facultative heterochromatin, is enriched in repressed genes, whereas euchromatin is enriched in active genes (103). From live-cell fluorescence microscopy as well as in vitro studies, segregation of heterochromatin has been linked to LLPS (42, 43, 44), although it has been argued that heterochromatin formation via bridging proteins like HP1 can also be explained by PPPS (57). The nucleus also harbors several distinct compartments enriched in proteins and RNA for specific functions (Fig. 2). Many compartments include chromatin, whereas some compartments are present in the interchromatin space. Compartments without chromatin therefore may not be directly dependent on the physical and chemical properties of the chromatin DNA. Compartments that include DNA and are dependent on DNA biochemistry include the nucleolus (104), Polycomb bodies (105,106), and transcription and replication factories (107, 108, 109, 110).
Figure 2.
Associative phase separation and the formation of membraneless organelles. (a) Under appropriate environmental conditions (concentration, temperature, solution ionic strength, etc.), oppositely charged polymers spontaneously separate into a solution phase that is depleted of the polymers and a condensed phase that is rich in the polymers. (b) This general phenomenon has been proposed as the driving force for the formation of several nuclear compartments that contain charged proteins and nucleic acids. The formation, dynamics, and stability of these compartments can be affected by not only the concentration levels but also the structural aspects of the constituent biomolecules. The figure includes diagrammatic structures of RNA depicted as hairpin and bulge structures in the RNA-enriched compartments. To see this figure in color, go online.
The nucleolus, the coacervate nature of which was described as early as the 1940s (69), now stands as a hallmark for LLPS of membraneless organelles in vivo. It is a prominently visible nuclear compartment where ribosomal genes (ribosomal DNA (rDNA)) are transcribed and ribosomal RNAs are processed (104). The nucleolus is formed around arrays of rDNA repeats termed nucleolar organizer regions (NORs). Although NORs themselves remain poorly understood regarding their sequence and genomic map, the telomeric side of NORs, termed distal junctions localized in perinucleolar heterochromatin, have been characterized (111,112). It has been suggested that distal junctions anchor rDNA to the perinucleolar heterochromatin (111) and thus could contribute to partitioning of the rDNA to the nucleolus. There are several recent studies providing insights into the connection between phase separation and the biochemistry of the nucleolus, reviewed in (113,114). However, questions regarding how the features of nucleolar DNA affects phase separation of the nucleolus still remain. For instance, how the sequence and local physical properties of DNA sequence elements of NORs play a role in the binding/partitioning of key proteins associated with NORs, such as the upstream binding factor (111), remain unexplored.
Polycomb bodies are sites in the nucleus, mostly associated with gene silencing, where Polycomb group (PcG) proteins are concentrated (28). Recently, it has been suggested that Polycomb bodies are assembled via phase separation of PcG proteins (115, 116, 117). Recruitment of PcG proteins is known to occur via binding to specific DNA sequences termed Polycomb repressive elements (PRE). However, PREs lack sequence homology and remain poorly characterized in mammals (27). It is thought that the specificity of recruited PcG proteins may be determined by transcription factors, chromatin modifications, and certain RNAs (118). In Drosophila, the PREs are often enriched in DNA motifs that are sequence specific to many transcription factors (28). In embryonic stem cells, transcriptionally inactive GC-rich DNA sequence elements (CpG islands) can initiate the recruitment of Polycomb repressive complex 2 (119). It remains to be investigated if and how the features of PREs contribute to phase separation of Polycomb bodies. PRE sequence features can include the GC content and distribution, propensity for chemical modifications, the number and PcG-protein-binding affinity of transcription-factor-binding DNA motifs, and sequence complementarity with RNA motifs enriched in the Polycomb bodies.
Other well-studied nuclear compartments include Cajal bodies (120), histone locus bodies (121), nuclear speckles (88), paraspeckles (122), and PML bodies (123). PML nuclear bodies typically do not contain RNA or DNA (123), whereas Cajal bodies, histone locus bodies, nuclear speckles, and paraspeckles are thought to nucleate via coding or noncoding RNA (124,125). The role of RNA in the formation of these nuclear bodies has been reviewed in (126). For discussions on recent studies implicating phase separation of specific proteins in the formation of these compartments, many reviews are available (113,126, 127, 128, 129). Depending on whether nucleic acids are required for the nucleation and growth of the compartments, their formation and stability can be governed by the nucleic acid secondary or tertiary structure, propensity for chemical modifications, and local mechanical properties (modulated by the sequence, presence of transient nicks, single-stranded loops, or bulges).
Chromosome conformation capture data and relationship between DNA sequence and phase separation of chromatin compartments and domains
Chromosome conformation capture experiments have enabled recognition of chromatin compartments and domains and advanced our knowledge on the hierarchical organization of the genome (Fig. 3; (130,131)). Chromatin compartmentalization, depicted via the contact maps obtained from such experiments, have been explained to occur via phase separation (93,94) using models of block copolymer phase separation driven by weak attractions (95). However, the sequence features of the genomic DNA that drive the phase separation of the chromatin compartments as inferred from chromosome conformation capture data are yet to be understood. In this section, we discuss how overlapping data sets analyzing sequence features of the genome with chromosome conformation capture data could provide insights into the role of DNA sequence in phase separation.
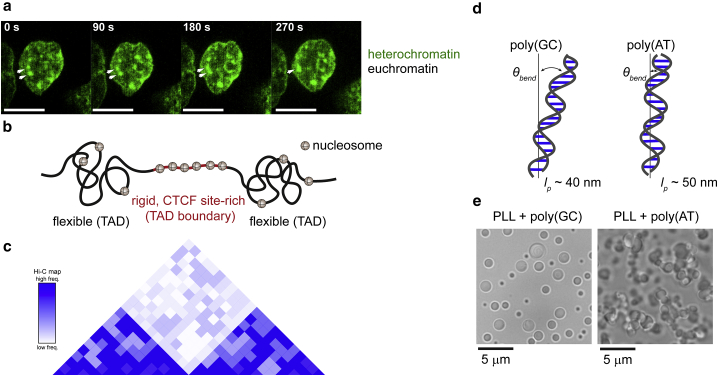
Figure 3.
Phase separation of chromatin and the role of DNA local mechanical properties. (a) The linker histone H1 forms liquid-like condensates that merge with neighboring condensates in HeLa nuclei, as evidenced by fluorescence imaging of GFP-tagged H1. The H1-condensates are colocalized with heterochromatin. The scale bar represents 5 μm. (b) On the submegabase scale, the chromatin has features called topologically associated domains (TADs), the boundary of which are enriched in CTCF binding sites flanked by closely spaced nucleosome arrays that increase the rigidity of chromatin. (c) Local organization of chromatin is revealed in Hi-C contact map data, which show regions of chromatin DNA that associate strongly and the regions that associate less frequently. (d) The persistence length (lp) of a property of the DNA as a polymer, quantifies the local flexibility of the DNA chain. Local flexibility is DNA sequence dependent. GC-rich DNA sequences are more flexible than AT-rich sequences. (e) Sequence-dependent flexibility tunes DNA phase behavior. More flexible DNA sequences in the presence of charged polymers like poly-L-lysine favor the formation of liquid-like droplets compared to more rigid DNA sequences. To see this figure in color, go online.
In Hi-C maps, plaid patterns of high-frequency and low-frequency interactions denote A- and B-compartments, respectively, which are now understood to be the broadly classified euchromatin and heterochromatin, respectively (132,133). Studies have shown that A/B-compartments can be further divided into subcompartments based on specific epigenetic marks (133). DNA sequence analysis shows that the A-compartments are GC rich, whereas B-compartments are AT rich (134). The A-compartments are also enriched in CpG dinucleotides and CCCTC-binding factor (CTCF) binding sites (134,135). Liu et al. found that high and low density of CG-islands (CGIs) are arranged alternately along the linear DNA sequence, referred to as forests and prairies for CGI-rich and CGI-poor domains, respectively (135). They showed that the structural features of the CGI forests and prairies overlap well with that of compartments and domains depicted via Hi-C data. Based on DNase sensitivity data, it has been proposed that interactions between the prairies drives their phase separation because of their high occupancy of histones (25,135). These studies thus suggest that insights into DNA-sequence-driven phase separation of chromatin can be obtained by utilizing chromosome conformation capture data.
Higher-resolution Hi-C experiments have allowed for detection of features in the submegabase scale such as chromatin loops and topologically associated domains (TADs) (130). TADs have been considered as physically isolated chromatin units in which clustered genes are often coregulated (136, 137, 138, 139). Although it is not fully understood how the genetic information guides TAD formation, there is evidence that certain DNA sequence elements are important in the mechanism. For example, correlating Hi-C data with DNA sequence analysis for CTCF binding sites and housekeeping genes shows that these elements are highly enriched at TAD boundaries (133,140,141). Such sites are found to be flanked by closely spaced nucleosome arrays (30). It has thus been hypothesized that the decreased flexibility of these high nucleosome occupancy sites is a potential mechanism of inhibition of inter-TAD interactions at the TAD boundaries (31). Furthermore, GC-rich TADs have been shown to have increased interchromosomal contacts and possible clustering behavior, attributed to “softer” mechanical properties of GC-rich domains (142). Although the segregation of A/B-compartments (euchromatin/heterochromatin) has been attributed to LLPS (42, 43, 44), the role of TADs in LLPS and vice versa is yet to be explored. We expect that future studies will be directed toward elucidating whether and how DNA sequence/structure plays a role in phase separation of chromatin compartments and domains.
How do repetitive DNA sequences contribute to phase separation?
Repetitive DNA sequences, which account for a large fraction of the genome (143, 144, 145), are now understood to have important structural and functional consequences (146,147). Many repetitive DNA sequences are found in the constitutive heterochromatin, where they are silenced as a part of the cell’s mechanism to maintain genome integrity (148). DNA repeats have been categorized as interspersed throughout the genome (for e.g., transposable elements and transfer RNA genes) and as tandem repeats (rDNA repeats in the nucleolus and satellite DNA in telomeres and centromeres) (144,149). As discussed earlier, LLPS has been implicated in the segregation of heterochromatin (42, 43, 44) as well as the formation of membraneless organelles enriched in repetitive DNA such as the nucleolus (69,113,114). Phase separation can cluster DNA elements that are distant in the linear sequence, allowing them to be in close physical proximity in three dimensions. Telomeres, which are repetitive DNA sequences at chromosomes ends, have been also shown to cluster in cells via phase separation (150,151).
Identical DNA sequences appear to have an inherent property to self-interact (152). This type of interaction is thought to enable recombination-independent pairing of homologous chromosomes (153). Assuming homology sensing between repetitive DNA sequences, Tang has proposed that repetitive DNA can drive chromatin folding and phase separation (26). In addition, high copy numbers and clustered distribution patterns of certain DNA repeats could facilitate the interactions between members in the same family of repeats, contributing to phase separation (26). It has also been shown that RNA-protein droplets in homologous chromosomes do not fuse if they contain different species of noncoding RNA (154,155). This suggests that the RNA sequence, which in turn reflects the DNA sequence where it accumulates, plays a role in pairing of homologous chromosomes.
Do local structural properties of nucleic acids play a role in phase separation?
Although the genomic DNA has the basic information on chromatin organization, in the context of in vivo phase separation, we have yet to understand how properties of the DNA itself plays a role. As discussed above, there are some hints as to how DNA sequence features could govern formation of chromatin compartments. Local structural properties of DNA could also contribute to the nucleation and maintenance of nuclear bodies assembled around chromatin segments. Although many recent studies have explored the role of protein sequence and structure in LLPS (156), there are relatively few studies (14,21) focused on such aspects of DNA. In vitro studies by Shakya et al. (14) have shown that local flexibility encoded by the DNA sequence can bias the phase behavior, with more flexible DNA sequences promoting LLPS and stability of resulting droplets against salt. This applies to hybridization-based changes in flexibility that increase the rigidity of the molecule as well as sequence-dependent flexibility of individual single or double strands (Fig. 3). In this section, we discuss the mechanical properties of DNA and how it can contribute to phase separation of DNA.
The persistence length (lp) of a polymer chain is defined as the length scale beyond which the polymer is strongly bent from thermal fluctuations (157). Under physiological conditions, double-stranded DNA (dsDNA) has a high lp of ∼50 nm (158). In contrast, the lp of single-stranded DNA (ssDNA) is <5 nm, making it a model flexible polymer (158). The large difference in local flexibility of DNA, depending on whether the DNA is single- or double-stranded, has been demonstrated to dictate phase behavior of DNA in presence of polycationic polymers (14,21) and histones (19). Experiments studying different ssDNA and dsDNA sequences further demonstrated that sequence-dependent local flexibility, not simply linear charge densities, modulate the phase behavior of DNA (14). Specifically, dsDNA helices rich in GC, which are more flexible than sequences rich in AT (159), were found to undergo LLPS more readily (14). Note, this trend extends to ssDNA (14), in which the polyT phase separates more readily than polyA because of the slightly larger lp of polyA (160). The results demonstrate that the free energy for complexation of two polyelectrolyte chains is partially offset by the bending energy of the chains (14,161). Indeed, molecular dynamics simulations have shown that upon complexation with oppositely charged polymers, flexible polyelectrolyte chains form dynamic clusters with higher density and structural correlation compared with rigid chains (162).
The physical properties of dsDNA have also been shown to be sensitive to modifications such as methylation (163,164). Methylation of the CpG nucleotide steps, which are enriched in human promotor sequences, was shown to significantly increase the local lp of the DNA (163), making nucleosome incorporation difficult. Subsequent work by Ha and co-workers (164) further confirmed that cytosine methylation decreased local DNA flexibility, thereby decreasing nucleosome stability. In addition, they found modifications of methylated cytosine, including hydroxymethylcytosine and formylcytosine (formed during the demethylation pathway), increased local DNA flexibility and nucleosome stability, demonstrating the complex role of nucleotide modifications in DNA mechanics. Recent in vitro studies have shown that polynucleosome chains can form liquid droplets, with the phase behavior being dependent on factors such as type of DNA-binding proteins (44) and internucleosome distance (165). Because the mechanical properties of DNA are linked with the formation, stability (166), and dynamics of the nucleosome core particle (167,168), we anticipate their effects on the phase separation of chromatin in vivo.
Although this review is focused on the phase separation of DNA and how its structural features can play a role, the discussion would perhaps be incomplete without discussing RNA phase separation. RNA, in contrast to DNA, can fold into complex three-dimensional structures with both single- and double-stranded characters (169). Many membraneless organelles are known to contain RNA. There are few studies examining the impact of the structural diversity of RNA on phase behavior. Nott and Baldwin (170) demonstrated that condensates formed from the N-terminus of the Ddx4 protein selectively partitioned flexible nucleic acids, including ssDNA and single-stranded RNA, as well as regulatory RNA and hairpins that are rich in single-stranded features, while destabilizing or excluding dsDNA and double-stranded RNA. Similarly, complexation of messenger RNA with the polyQ protein Whi3 has been shown to induce sequence-specific structural changes of the messenger RNA (22). In contrast, NMR experiments have shown that the structure and dynamics of a hairpin RNA are not influenced by complexation with polycationic polymers (171). Vale and co-workers have demonstrated that repeat-containing RNA phase separate in the absence of proteins via basepairing, ultimately forming gels at a critical hybridization content (172). Many processes in cells, such as transcription and DNA damage (173, 174, 175, 176), can result in large changes in the structure and mechanical properties of the localized DNA and RNA and alter interactions with binding partners. Therefore, it is likely that the structural features of the nucleic acids affect phase behavior associated with such processes. Although it is challenging to directly measure structural changes in vivo, the development of new approaches (177) is necessary to advance our understanding of the role of nucleic acids structure in the phase separation of nuclear compartments.
Conclusions
The phenomenon of associative phase separation of biological molecules has a long and rich history spanning more than a century. The past decade has brought many new discoveries and insights that have highlighted the importance of phase separation in biology. The field has benefited greatly from interdisciplinary knowledge and expertise because key insights have come from communities ranging from cell biology to polyelectrolyte/polymer physics. To date, most studies on phase separation have focused on proteins. However, studies implicating the role of nucleic acid sequence and structural features are also emerging. We anticipate that future investigations on how nucleic acid mechanical properties, sequence, and the genomic context manifest in phase separation will enhance our understanding of membraneless intracellular organization and function.
acknowledgments
The work was supported by the Korean Institute for Basic Science, project code IBS-R020-D1.
Editor: Meyer Jackson.
Contributor Information
John T. King, Email: jtking@unist.ac.kr.
Anisha Shakya, Email: shanisha@unist.ac.kr.
References
- 1.Diekmann Y., Pereira-Leal J.B. Evolution of intracellular compartmentalization. Biochem. J. 2013;449:319–331. doi: 10.1042/BJ20120957. [DOI] [PubMed] [Google Scholar]
- 2.Brangwynne C.P., Eckmann C.R., Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 3.Hyman A.A., Weber C.A., Jülicher F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- 4.Banani S.F., Lee H.O., Rosen M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin Y., Brangwynne C.P. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4832. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 6.Uversky V.N. Protein intrinsic disorder-based liquid-liquid phase transitions in biological systems: complex coacervates and membrane-less organelles. Adv. Colloid Interface Sci. 2017;239:97–114. doi: 10.1016/j.cis.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 7.You K., Huang Q., Li T. PhaSepDB: a database of liquid-liquid phase separation related proteins. Nucleic Acids Res. 2020;48:D354–D359. doi: 10.1093/nar/gkz847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning W., Guo Y., Xue Y. DrLLPS: a data resource of liquid-liquid phase separation in eukaryotes. Nucleic Acids Res. 2020;48:D288–D295. doi: 10.1093/nar/gkz1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mészáros B., Erdős G., Pancsa R. PhaSePro: the database of proteins driving liquid-liquid phase separation. Nucleic Acids Res. 2020;48:D360–D367. doi: 10.1093/nar/gkz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q., Peng X., Zhang Z. LLPSDB: a database of proteins undergoing liquid-liquid phase separation in vitro. Nucleic Acids Res. 2020;48:D320–D327. doi: 10.1093/nar/gkz778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nott T.J., Petsalaki E., Baldwin A.J. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dignon G.L., Zheng W., Mittal J. Temperature-controlled liquid-liquid phase separation of disordered proteins. ACS Cent. Sci. 2019;5:821–830. doi: 10.1021/acscentsci.9b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harami G.M., Kovács Z.J., Kovács M. Phase separation by ssDNA binding protein controlled via protein-protein and protein-DNA interactions. Proc. Natl. Acad. Sci. USA. 2020;117:26206–26217. doi: 10.1073/pnas.2000761117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakya A., King J.T. DNA local-flexibility-dependent assembly of phase-separated liquid droplets. Biophys. J. 2018;115:1840–1847. doi: 10.1016/j.bpj.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinbreck F., de Vries R., de Kruif C.G. Complex coacervation of whey proteins and gum Arabic. Biomacromolecules. 2003;4:293–303. doi: 10.1021/bm025667n. [DOI] [PubMed] [Google Scholar]
- 16.Last M.G.F., Deshpande S., Dekker C. pH-controlled coacervate-membrane interactions within liposomes. ACS Nano. 2020;14:4487–4498. doi: 10.1021/acsnano.9b10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y., Protter D.S., Parker R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell. 2015;60:208–219. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H., Elbaum-Garfinkle S., Gladfelter A.S. RNA controls polyQ protein phase transitions. Mol. Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakya A., King J.T. Non-Fickian molecular transport in protein-DNA droplets. ACS Macro Lett. 2018;7:1220–1225. doi: 10.1021/acsmacrolett.8b00565. [DOI] [PubMed] [Google Scholar]
- 20.Patel A., Malinovska L., Hyman A.A. ATP as a biological hydrotrope. Science. 2017;356:753–756. doi: 10.1126/science.aaf6846. [DOI] [PubMed] [Google Scholar]
- 21.Vieregg J.R., Lueckheide M., Tirrell M.V. Oligonucleotide-peptide complexes: phase control by hybridization. J. Am. Chem. Soc. 2018;140:1632–1638. doi: 10.1021/jacs.7b03567. [DOI] [PubMed] [Google Scholar]
- 22.Langdon E.M., Qiu Y., Gladfelter A.S. mRNA structure determines specificity of a polyQ-driven phase separation. Science. 2018;360:922–927. doi: 10.1126/science.aar7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L., Hu M., Li P. Rett syndrome-causing mutations compromise MeCP2-mediated liquid-liquid phase separation of chromatin. Cell Res. 2020;30:393–407. doi: 10.1038/s41422-020-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang H., Yoo J., Kim H. Sequence-dependent DNA condensation as a driving force of DNA phase separation. Nucleic Acids Res. 2018;46:9401–9413. doi: 10.1093/nar/gky639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan H., Yang Y., Gao Y.Q. Chromatin structure changes during various processes from a DNA sequence view. Curr. Opin. Struct. Biol. 2020;62:1–8. doi: 10.1016/j.sbi.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Tang S.J. Potential role of phase separation of repetitive DNA in chromosomal organization. Genes (Basel) 2017;8:1–8. doi: 10.3390/genes8100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer M., Trupke J., Ringrose L. The quest for mammalian Polycomb response elements: are we there yet? Chromosoma. 2016;125:471–496. doi: 10.1007/s00412-015-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Entrevan M., Schuettengruber B., Cavalli G. Regulation of genome architecture and function by Polycomb proteins. Trends Cell Biol. 2016;26:511–525. doi: 10.1016/j.tcb.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Shrinivas K., Sabari B.R., Chakraborty A.K. Enhancer features that drive formation of transcriptional condensates. Mol. Cell. 2019;75:549–561.e7. doi: 10.1016/j.molcel.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valouev A., Johnson S.M., Sidow A. Determinants of nucleosome organization in primary human cells. Nature. 2011;474:516–520. doi: 10.1038/nature10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixon J.R., Gorkin D.U., Ren B. Chromatin Domains: the unit of chromosome organization. Mol. Cell. 2016;62:668–680. doi: 10.1016/j.molcel.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dignon G.L., Best R.B., Mittal J. Biomolecular phase separation: from molecular driving forces to macroscopic properties. Annu. Rev. Phys. Chem. 2020;71:53–75. doi: 10.1146/annurev-physchem-071819-113553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J.M., Holehouse A.S., Pappu R.V. Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys. 2020;49:107–133. doi: 10.1146/annurev-biophys-121219-081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Gennes P.G. Cornell University Press; Ithica, NY: 1979. Scaling Concepts in Polymer Physics. [Google Scholar]
- 35.Overbeek J.T.G., Voorn M.J. Phase separation in polyelectrolyte solutions. Theory of complex coacervation. J. Cell. Physiol. Suppl. 1957;49(Suppl 1):7–26. [PubMed] [Google Scholar]
- 36.Nakajima A., Sato H. Phase relationships of an equivalent mixture of sulfated polyvinyl alcohol and aminoacetalyzed polyvinyl alcohol in microsalt aqueous-solution. Biopolymers. 1972;11:1345–1355. [Google Scholar]
- 37.Spruijt E., Westphal A.H., van der Gucht J. Binodal compositions of polyeletrolyte complexes. Macromolecules. 2010;43:6476–6484. [Google Scholar]
- 38.Sing C.E. Development of the modern theory of polymeric complex coacervation. Adv. Colloid Interface Sci. 2017;239:2–16. doi: 10.1016/j.cis.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y.H., Forman-Kay J.D., Chan H.S. Sequence-specific polyampholyte phase separation in membraneless organelles. Phys. Rev. Lett. 2016;117:178101. doi: 10.1103/PhysRevLett.117.178101. [DOI] [PubMed] [Google Scholar]
- 40.Wang J., Choi J.M., Hyman A.A. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 2018;174:688–699.e16. doi: 10.1016/j.cell.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin E.W., Holehouse A.S., Mittag T. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science. 2020;367:694–699. doi: 10.1126/science.aaw8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larson A.G., Elnatan D., Narlikar G.J. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature. 2017;547:236–240. doi: 10.1038/nature22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strom A.R., Emelyanov A.V., Karpen G.H. Phase separation drives heterochromatin domain formation. Nature. 2017;547:241–245. doi: 10.1038/nature22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shakya A., Park S., King J.T. Liquid-liquid phase separation of histone proteins in cells: role in chromatin organization. Biophys. J. 2020;118:753–764. doi: 10.1016/j.bpj.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boija A., Klein I.A., Young R.A. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 2018;175:1842–1855.e16. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabari B.R., Dall’Agnese A., Young R.A. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361:eaar3958. doi: 10.1126/science.aar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langdon E.M., Gladfelter A.S. A new lens for RNA localization: liquid-liquid phase separation. Annu. Rev. Microbiol. 2018;72:255–271. doi: 10.1146/annurev-micro-090817-062814. [DOI] [PubMed] [Google Scholar]
- 48.Oshidari R., Huang R., Mekhail K. DNA repair by Rad52 liquid droplets. Nat. Commun. 2020;11:695. doi: 10.1038/s41467-020-14546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilic S., Lezaja A., Altmeyer M. Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. EMBO J. 2019;38:e101379. doi: 10.15252/embj.2018101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li P., Banjade S., Rosen M.K. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chong P.A., Forman-Kay J.D. Liquid-liquid phase separation in cellular signaling systems. Curr. Opin. Struct. Biol. 2016;41:180–186. doi: 10.1016/j.sbi.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Du M., Chen Z.J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoo H., Triandafillou C., Drummond D.A. Cellular sensing by phase separation: using the process, not just the products. J. Biol. Chem. 2019;294:7151–7159. doi: 10.1074/jbc.TM118.001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alberti S., Gladfelter A., Mittag T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell. 2019;176:419–434. doi: 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McSwiggen D.T., Mir M., Tjian R. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev. 2019;33:1619–1634. doi: 10.1101/gad.331520.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mir M., Bickmore W., Narlikar G. Chromatin topology, condensates and gene regulation: shifting paradigms or just a phase? Development. 2019;146:dev182766. doi: 10.1242/dev.182766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erdel F., Rippe K. Formation of chromatin subcompartments by phase separation. Biophys. J. 2018;114:2262–2270. doi: 10.1016/j.bpj.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong S., Dugast-Darzacq C., Tjian R. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 2018;361:eaar2555. doi: 10.1126/science.aar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kossel A. Uber die basischen stoffen des zellkerns. Hoppe-Seyler’s Z. Phys. Chem. 1897;22:176–187. [Google Scholar]
- 60.Tsuchida E., Abe K. Springer; Berlin, Heidelberg: 1982. Interactions Between Macromolecules in Solution and Intermacromolecular Complexes; pp. 1–119. [Google Scholar]
- 61.Evreinova T.N. Nauka; Moscow, Russia: 1966. Concentration of Matter and Action of Enzymes in Coacervates. [Google Scholar]
- 62.Butschli V.O. Adam and Charles Black; London: 1894. Investigations on Microscopic Foams and on Protoplasm. [Google Scholar]
- 63.Andrews E.A. On the structure of the protoplasm. Science. 1895;2:893–899. [Google Scholar]
- 64.Wilson E.B. The structure of protoplasm. Science. 1899;10:33–45. doi: 10.1126/science.10.237.33. [DOI] [PubMed] [Google Scholar]
- 65.Montgomery T.S.H., Jr. Comparative cytological studies with especial regard to the morphology of the nucleolus. J. Morphol. 1898;25:265–582. [Google Scholar]
- 66.Veis A. A review of the early development of the thermodynamics of the complex coacervation phase separation. Adv. Colloid Interface Sci. 2011;167:2–11. doi: 10.1016/j.cis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Jong H.G.B. Die koazervation und ihre bedeutung für die biologie. Protoplasma. 1932;15:110–173. [Google Scholar]
- 68.Frey-Wyssling A. Elsevier Publishing; London: 1948. Submicroscopic Morphology of Protoplasm and Its Derivatives. [Google Scholar]
- 69.Ehrenberg L. Influence of temperature on the nucleolus and its coacervate nature. Hereditas. 1946;32:407–418. doi: 10.1111/j.1601-5223.1946.tb02783.x. [DOI] [PubMed] [Google Scholar]
- 70.Oparin A.I. The Macmillian Company; New York: 1938. The Origin of Life. [Google Scholar]
- 71.Leforestier A., Livolant F. Liquid crystalline ordering of nucleosome core particles under macromolecular crowding conditions: evidence for a discotic columnar hexagonal phase. Biophys. J. 1997;73:1771–1776. doi: 10.1016/S0006-3495(97)78207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teif V.B., Bohinc K. Condensed DNA: condensing the concepts. Prog. Biophys. Mol. Biol. 2011;105:208–222. doi: 10.1016/j.pbiomolbio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Lasic D.D., Papahadjopoulos D., Podgornik R. Polymorphism of lipids, nucleic acids, and their interactions. In: Kabanov A.V., Felgner P.L., Seymour L.W., editors. Self-Assembling Complexes for Gene Delivery: From Laboratory to Clinical Trial. John Wiley and Sons; 1998. pp. 3–26. [Google Scholar]
- 74.Livolant F., Mangenot S., Durand D. Are liquid crystalline properties of nucleosomes involved in chromosome structure and dynamics? Philos. Trans. A Math. Phys. Eng. Sci. 2006;364:2615–2633. doi: 10.1098/rsta.2006.1843. [DOI] [PubMed] [Google Scholar]
- 75.Wong J.T.Y. Architectural organization of dinoflagellate liquid crystalline chromosomes. Microorganisms. 2019;7:1–10. doi: 10.3390/microorganisms7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finch J.T., Klug A. Solenoidal model for superstructure in chromatin. Proc. Natl. Acad. Sci. USA. 1976;73:1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson P.J., Fairall L., Rhodes D. EM measurements define the dimensions of the “30-nm” chromatin fiber: evidence for a compact, interdigitated structure. Proc. Natl. Acad. Sci. USA. 2006;103:6506–6511. doi: 10.1073/pnas.0601212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maeshima K., Hihara S., Eltsov M. Chromatin structure: does the 30-nm fibre exist in vivo? Curr. Opin. Cell Biol. 2010;22:291–297. doi: 10.1016/j.ceb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 79.Tremethick D.J. Higher-order structures of chromatin: the elusive 30 nm fiber. Cell. 2007;128:651–654. doi: 10.1016/j.cell.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 80.McDowall A.W., Smith J.M., Dubochet J. Cryo-electron microscopy of vitrified chromosomes in situ. EMBO J. 1986;5:1395–1402. doi: 10.1002/j.1460-2075.1986.tb04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maeshima K., Ide S., Sasai M. Liquid-like behavior of chromatin. Curr. Opin. Genet. Dev. 2016;37:36–45. doi: 10.1016/j.gde.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Misteli T., Gunjan A., Brown D.T. Dynamic binding of histone H1 to chromatin in living cells. Nature. 2000;408:877–881. doi: 10.1038/35048610. [DOI] [PubMed] [Google Scholar]
- 83.Lever M.A., Th’ng J.P.H., Hendzel M.J. Rapid exchange of histone H1.1 on chromatin in living human cells. Nature. 2000;408:873–876. doi: 10.1038/35048603. [DOI] [PubMed] [Google Scholar]
- 84.Contreras A., Hale T.K., Herrera R.E. The dynamic mobility of histone H1 is regulated by cyclin/CDK phosphorylation. Mol. Cell. Biol. 2003;23:8626–8636. doi: 10.1128/MCB.23.23.8626-8636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bernas T., Brutkowski W., Dobrucki J. Spatial heterogeneity of dynamics of H1 linker histone. Eur. Biophys. J. 2014;43:287–300. doi: 10.1007/s00249-014-0962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Platani M., Goldberg I., Lamond A.I. In vivo analysis of Cajal body movement, separation, and joining in live human cells. J. Cell Biol. 2000;151:1561–1574. doi: 10.1083/jcb.151.7.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weidtkamp-Peters S., Lenser T., Hemmerich P. Dynamics of component exchange at PML nuclear bodies. J. Cell Sci. 2008;121:2731–2743. doi: 10.1242/jcs.031922. [DOI] [PubMed] [Google Scholar]
- 88.Spector D.L., Lamond A.I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011;3:a000646. doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ficz G., Heintzmann R., Arndt-Jovin D.J. Polycomb group protein complexes exchange rapidly in living Drosophila. Development. 2005;132:3963–3976. doi: 10.1242/dev.01950. [DOI] [PubMed] [Google Scholar]
- 90.Walter H., Brooks D.E. Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Lett. 1995;361:135–139. doi: 10.1016/0014-5793(95)00159-7. [DOI] [PubMed] [Google Scholar]
- 91.Iborra F.J. Can visco-elastic phase separation, macromolecular crowding and colloidal physics explain nuclear organisation? Theor. Biol. Med. Model. 2007;4:15. doi: 10.1186/1742-4682-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanaka H., Nishikawa Y. Viscoelastic phase separation of protein solutions. Phys. Rev. Lett. 2005;95:078103. doi: 10.1103/PhysRevLett.95.078103. [DOI] [PubMed] [Google Scholar]
- 93.Haddad N., Jost D., Vaillant C. Perspectives: using polymer modeling to understand the formation and function of nuclear compartments. Chromosome Res. 2017;25:35–50. doi: 10.1007/s10577-016-9548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nuebler J., Fudenberg G., Mirny L.A. Chromatin organization by an interplay of loop extrusion and compartmental segregation. Proc. Natl. Acad. Sci. USA. 2018;115:E6697–E6706. doi: 10.1073/pnas.1717730115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lohse D.J., Hadjichristidis N. Microphase separation in block copolymers. Curr. Opin. Colloid Interface Sci. 1997;2:171–176. [Google Scholar]
- 96.Machida S., Takizawa Y., Kurumizaka H. Structural basis of heterochromatin formation by human HP1. Mol. Cell. 2018;69:385–397.e8. doi: 10.1016/j.molcel.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Zhou K., Gaullier G., Luger K. Nucleosome structure and dynamics are coming of age. Nat. Struct. Mol. Biol. 2019;26:3–13. doi: 10.1038/s41594-018-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fyodorov D.V., Zhou B.R., Bai Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat. Rev. Mol. Cell Biol. 2018;19:192–206. doi: 10.1038/nrm.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kempfer R., Pombo A. Methods for mapping 3D chromosome architecture. Nat. Rev. Genet. 2020;21:207–226. doi: 10.1038/s41576-019-0195-2. [DOI] [PubMed] [Google Scholar]
- 100.Cremer T., Cremer M. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2010;2:a003889. doi: 10.1101/cshperspect.a003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fritz A.J., Sehgal N., Berezney R. Chromosome territories and the global regulation of the genome. Genes Chromosomes Cancer. 2019;58:407–426. doi: 10.1002/gcc.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Passarge E. Emil Heitz and the concept of heterochromatin: longitudinal chromosome differentiation was recognized fifty years ago. Am. J. Hum. Genet. 1979;31:106–115. [PMC free article] [PubMed] [Google Scholar]
- 103.Allshire R.C., Madhani H.D. Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol. 2018;19:229–244. doi: 10.1038/nrm.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boisvert F.M., van Koningsbruggen S., Lamond A.I. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 105.Smigová J., Juda P., Raška I. Structural basis of polycomb bodies. Folia Biol. (Praha) 2014;60(Suppl 1):13–20. [PubMed] [Google Scholar]
- 106.Pirrotta V., Li H.B. A view of nuclear Polycomb bodies. Curr. Opin. Genet. Dev. 2012;22:101–109. doi: 10.1016/j.gde.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cook P.R. The organization of replication and transcription. Science. 1999;284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- 108.Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 109.Fraser P., Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–417. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- 110.Cisse I.I., Izeddin I., Darzacq X. Real-time dynamics of RNA polymerase II clustering in live human cells. Science. 2013;341:664–667. doi: 10.1126/science.1239053. [DOI] [PubMed] [Google Scholar]
- 111.McStay B. Nucleolar organizer regions: genomic ‘dark matter’ requiring illumination. Genes Dev. 2016;30:1598–1610. doi: 10.1101/gad.283838.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Floutsakou I., Agrawal S., McStay B. The shared genomic architecture of human nucleolar organizer regions. Genome Res. 2013;23:2003–2012. doi: 10.1101/gr.157941.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Correll C.C., Bartek J., Dundr M. The nucleolus: a multiphase condensate balancing ribosome synthesis and translational capacity in health, aging and ribosomopathies. Cells. 2019;8:1–19. doi: 10.3390/cells8080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Latonen L. Phase-to-phase with nucleoli - stress responses, protein aggregation and novel roles of RNA. Front. Cell. Neurosci. 2019;13:151. doi: 10.3389/fncel.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Plys A.J., Davis C.P., Kingston R.E. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 2019;33:799–813. doi: 10.1101/gad.326488.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tatavosian R., Kent S., Ren X. Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. J. Biol. Chem. 2019;294:1451–1463. doi: 10.1074/jbc.RA118.006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qu W., Wang Z., Zhang H. Phase separation of the C. elegans Polycomb protein SOP-2 is modulated by RNA and sumoylation. Protein Cell. 2020;11:202–207. doi: 10.1007/s13238-019-00680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schuettengruber B., Bourbon H.M., Cavalli G. Genome regulation by polycomb and trithorax: 70 years and counting. Cell. 2017;171:34–57. doi: 10.1016/j.cell.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 119.Mendenhall E.M., Koche R.P., Bernstein B.E. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genet. 2010;6:e1001244. doi: 10.1371/journal.pgen.1001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sawyer I.A., Sturgill D., Dundr M. Cajal body function in genome organization and transcriptome diversity. BioEssays. 2016;38:1197–1208. doi: 10.1002/bies.201600144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marzluff W.F., Koreski K.P. Birth and death of histone mRNAs. Trends Genet. 2017;33:745–759. doi: 10.1016/j.tig.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fox A.H., Lamond A.I. Paraspeckles. Cold Spring Harb. Perspect. Biol. 2010;2:a000687. doi: 10.1101/cshperspect.a000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lång A., Lång E., Bøe S.O. PML bodies in mitosis. Cells. 2019;8:893. doi: 10.3390/cells8080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matera A.G., Izaguire-Sierra M., Rajendra T.K. Nuclear bodies: random aggregates of sticky proteins or crucibles of macromolecular assembly? Dev. Cell. 2009;17:639–647. doi: 10.1016/j.devcel.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shevtsov S.P., Dundr M. Nucleation of nuclear bodies by RNA. Nat. Cell Biol. 2011;13:167–173. doi: 10.1038/ncb2157. [DOI] [PubMed] [Google Scholar]
- 126.Sawyer I.A., Sturgill D., Dundr M. Membraneless nuclear organelles and the search for phases within phases. Wiley Interdiscip. Rev. RNA. 2019;10:e1514. doi: 10.1002/wrna.1514. [DOI] [PubMed] [Google Scholar]
- 127.Lesne A., Baudement M.-O., Forné T. Exploring mammalian genome within phase-separated nuclear bodies: experimental methods and implications for gene expression. Genes (Basel) 2019;10:1–13. doi: 10.3390/genes10121049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.A P., Weber S.C. Evidence for and against liquid-liquid phase separation in the nucleus. Noncoding RNA. 2019;5:1–14. doi: 10.3390/ncrna5040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sabari B.R., Dall’Agnese A., Young R.A. Biomolecular condensates in the nucleus. Trends Biochem. Sci. 2020;45:961–977. doi: 10.1016/j.tibs.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sati S., Cavalli G. Chromosome conformation capture technologies and their impact in understanding genome function. Chromosoma. 2017;126:33–44. doi: 10.1007/s00412-016-0593-6. [DOI] [PubMed] [Google Scholar]
- 131.Dekker J., Marti-Renom M.A., Mirny L.A. Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nat. Rev. Genet. 2013;14:390–403. doi: 10.1038/nrg3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lieberman-Aiden E., van Berkum N.L., Dekker J. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rao S.S.P., Huntley M.H., Aiden E.L. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie W.J., Meng L., Gao Y.Q. Structural modeling of chromatin integrates genome features and reveals chromosome folding principle. Sci. Rep. 2017;7:2818. doi: 10.1038/s41598-017-02923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu S., Zhang L., Gao Y.Q. From 1D sequence to 3D chromatin dynamics and cellular functions: a phase separation perspective. Nucleic Acids Res. 2018;46:9367–9383. doi: 10.1093/nar/gky633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Le Dily F., Baù D., Beato M. Distinct structural transitions of chromatin topological domains correlate with coordinated hormone-induced gene regulation. Genes Dev. 2014;28:2151–2162. doi: 10.1101/gad.241422.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhan Y., Mariani L., Giorgetti L. Reciprocal insulation analysis of Hi-C data shows that TADs represent a functionally but not structurally privileged scale in the hierarchical folding of chromosomes. Genome Res. 2017;27:479–490. doi: 10.1101/gr.212803.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramírez F., Bhardwaj V., Manke T. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat. Commun. 2018;9:189. doi: 10.1038/s41467-017-02525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nora E.P., Lajoie B.R., Heard E. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dixon J.R., Selvaraj S., Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Phillips-Cremins J.E., Corces V.G. Chromatin insulators: linking genome organization to cellular function. Mol. Cell. 2013;50:461–474. doi: 10.1016/j.molcel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jabbari K., Chakraborty M., Wiehe T. DNA sequence-dependent chromatin architecture and nuclear hubs formation. Sci. Rep. 2019;9:14646. doi: 10.1038/s41598-019-51036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Treangen T.J., Salzberg S.L. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat. Rev. Genet. 2011;13:36–46. doi: 10.1038/nrg3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Richard G.F., Kerrest A., Dujon B. Comparative genomics and molecular dynamics of DNA repeats in eukaryotes. Microbiol. Mol. Biol. Rev. 2008;72:686–727. doi: 10.1128/MMBR.00011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Koning A.P., Gu W., Pollock D.D. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011;7:e1002384. doi: 10.1371/journal.pgen.1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khristich A.N., Mirkin S.M. On the wrong DNA track: molecular mechanisms of repeat-mediated genome instability. J. Biol. Chem. 2020;295:4134–4170. doi: 10.1074/jbc.REV119.007678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hall A.C., Ostrowski L.A., Mekhail K. Repetitive DNA loci and their modulation by the non-canonical nucleic acid structures R-loops and G-quadruplexes. Nucleus. 2017;8:162–181. doi: 10.1080/19491034.2017.1292193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Janssen A., Colmenares S.U., Karpen G.H. Heterochromatin: guardian of the genome. Annu. Rev. Cell Dev. Biol. 2018;34:265–288. doi: 10.1146/annurev-cellbio-100617-062653. [DOI] [PubMed] [Google Scholar]
- 149.Paço A., Freitas R., Vieira-da-Silva A. Conversion of DNA sequences: from a transposable element to a tandem repeat or to a gene. Genes (Basel) 2019;10:1014. doi: 10.3390/genes10121014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Min J., Wright W.E., Shay J.W. Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Genes Dev. 2019;33:814–827. doi: 10.1101/gad.324905.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H., Zhao R., Lampson M.A. Nuclear body phase separation drives telomere clustering in ALT cancer cells. Mol. Biol. Cell. 2020:2048–2056. doi: 10.1091/mbc.E19-10-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mazur A.K., Nguyen T.S., Gladyshev E. Direct homologous dsDNA-dsDNA pairing: how, where, and why? J. Mol. Biol. 2020;432:737–744. doi: 10.1016/j.jmb.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 153.Gladyshev E., Kleckner N. Recombination-independent recognition of DNA homology for repeat-induced point mutation. Curr. Genet. 2017;63:389–400. doi: 10.1007/s00294-016-0649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ding D.Q., Okamasa K., Hiraoka Y. Chromosome-associated RNA-protein complexes promote pairing of homologous chromosomes during meiosis in Schizosaccharomyces pombe. Nat. Commun. 2019;10:5598. doi: 10.1038/s41467-019-13609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hiraoka Y. Phase separation drives pairing of homologous chromosomes. Curr. Genet. 2020;66:881–887. doi: 10.1007/s00294-020-01077-9. [DOI] [PubMed] [Google Scholar]
- 156.Weber S.C. Sequence-encoded material properties dictate the structure and function of nuclear bodies. Curr. Opin. Cell Biol. 2017;46:62–71. doi: 10.1016/j.ceb.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 157.Barrat J.-L., Joanny F. Theory of polyelectrolyte solutions. In: Prigogine I., Rice S.A., editors. Advances in Chemical Physics. John Wiley and Sons; 1996. pp. 1–66. [Google Scholar]
- 158.Camunas-Soler J., Ribezzi-Crivellari M., Ritort F. Elastic properties of nucleic acids by single-molecule force spectroscopy. Annu. Rev. Biophys. 2016;45:65–84. doi: 10.1146/annurev-biophys-062215-011158. [DOI] [PubMed] [Google Scholar]
- 159.Geggier S., Vologodskii A. Sequence dependence of DNA bending rigidity. Proc. Natl. Acad. Sci. USA. 2010;107:15421–15426. doi: 10.1073/pnas.1004809107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mills J.B., Vacano E., Hagerman P.J. Flexibility of single-stranded DNA: use of gapped duplex helices to determine the persistence lengths of poly(dT) and poly(dA) J. Mol. Biol. 1999;285:245–257. doi: 10.1006/jmbi.1998.2287. [DOI] [PubMed] [Google Scholar]
- 161.André A.A.M., Spruijt E. Rigidity rules in DNA droplets: nucleic acid flexibility affects model membraneless organelles. Biophys. J. 2018;115:1837–1839. doi: 10.1016/j.bpj.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shakya A., Girard M., de la Cruz M.O. Role of chain flexibility in asymmetric polyelectrolyte complexation in salt solutions. Macromolecules. 2020;53:1258–1269. [Google Scholar]
- 163.Pérez A., Castellazzi C.L., Orozco M. Impact of methylation on the physical properties of DNA. Biophys. J. 2012;102:2140–2148. doi: 10.1016/j.bpj.2012.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ngo T.T.M., Yoo J., Ha T. Effects of cytosine modifications on DNA flexibility and nucleosome mechanical stability. Nat. Commun. 2016;7:10813. doi: 10.1038/ncomms10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gibson B.A., Doolittle L.K., Rosen M.K. Organization of chromatin by intrinsic and regulated phase separation. Cell. 2019;179:470–484.e21. doi: 10.1016/j.cell.2019.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chua E.Y., Vasudevan D., Davey C.A. The mechanics behind DNA sequence-dependent properties of the nucleosome. Nucleic Acids Res. 2012;40:6338–6352. doi: 10.1093/nar/gks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ngo T.T., Zhang Q., Ha T. Asymmetric unwrapping of nucleosomes under tension directed by DNA local flexibility. Cell. 2015;160:1135–1144. doi: 10.1016/j.cell.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mauney A.W., Tokuda J.M., Pollack L. Local DNA sequence controls asymmetry of DNA unwrapping from nucleosome core particles. Biophys. J. 2018;115:773–781. doi: 10.1016/j.bpj.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Holbrook S.R. Structural principles from large RNAs. Annu. Rev. Biophys. 2008;37:445–464. doi: 10.1146/annurev.biophys.36.040306.132755. [DOI] [PubMed] [Google Scholar]
- 170.Nott T.J., Craggs T.D., Baldwin A.J. Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat. Chem. 2016;8:569–575. doi: 10.1038/nchem.2519. [DOI] [PubMed] [Google Scholar]
- 171.Shakya A., Dougherty C.A., Banaszak Holl M.M. Rapid exchange between free and bound states in RNA-dendrimer polyplexes: implications on the mechanism of delivery and release. Biomacromolecules. 2016;17:154–164. doi: 10.1021/acs.biomac.5b01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jain A., Vale R.D. RNA phase transitions in repeat expansion disorders. Nature. 2017;546:243–247. doi: 10.1038/nature22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hauer M.H., Seeber A., Gasser S.M. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nat. Struct. Mol. Biol. 2017;24:99–107. doi: 10.1038/nsmb.3347. [DOI] [PubMed] [Google Scholar]
- 174.Miné-Hattab J., Recamier V., Darzacq X. Multi-scale tracking reveals scale-dependent chromatin dynamics after DNA damage. Mol. Biol. Cell. 2017;28:3323–3332. doi: 10.1091/mbc.E17-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Herbert S., Brion A., Zimmer C. Chromatin stiffening underlies enhanced locus mobility after DNA damage in budding yeast. EMBO J. 2017;36:2595–2608. doi: 10.15252/embj.201695842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lavelle C. Pack, unpack, bend, twist, pull, push: the physical side of gene expression. Curr. Opin. Genet. Dev. 2014;25:74–84. doi: 10.1016/j.gde.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 177.Shakya A., King J.T. Modern optical microscopy methods to study biomolecular condensates. Curr. Opin. Colloid Interface Sci. 2021 doi: 10.1016/j.cocis.2021.101421. PUBLISHED ONLINE FEBRUARY 3, 2021. [DOI] [Google Scholar]