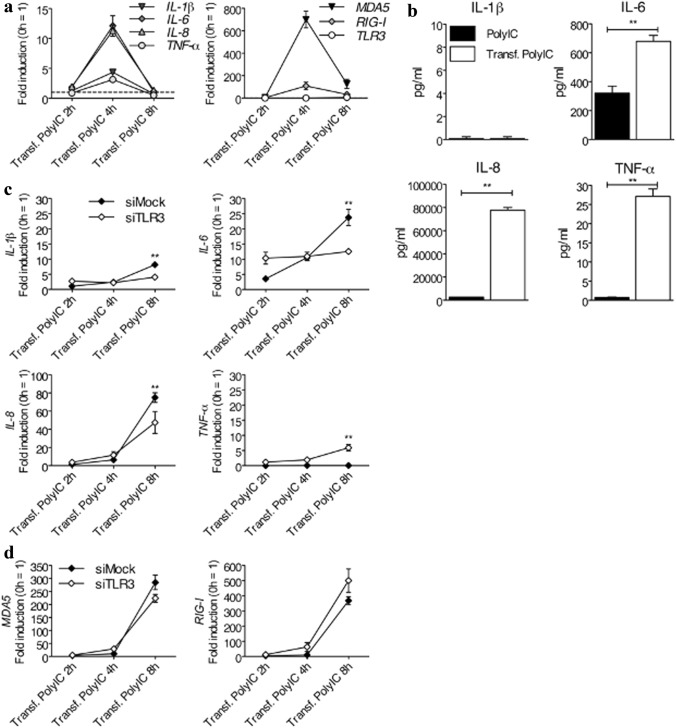
Fig. 4.
Human CF stimulated with transfected PolyIC produce higher levels of pro-inflammatory cytokines than after stimulation with untransfected PolyIC. (a) Human CF were stimulated for 2, 4, and 8 h with 1 ng/ml of intracellularly transfected PolyIC. Transcription of IL-1β, IL-6, IL-8, and TNF-α (left panel) and transcription of MDA5 and RIG-I, but not of TLR3 (right panel), all determined by real-time RT-PCR, was highly increased. (b) Human CF were stimulated for 24 h with 1 ng/ml PolyIC added to the medium to stimulate membrane-bound receptors, such as TLR3, or with 1 ng/ml of intracellularly transfected PolyIC to stimulate cytosolic receptors, such as MDA5 and RIG-I. IL-6, IL-8, and TNF-α, but not IL-1β, measured at the protein level in cell-free supernatants, were significantly higher after cytosolic receptors stimulation (transfected PolyIC) than after stimulation of membrane-bound receptor (PolyIC). (c-d) Knockdown of TLR3 with siRNA in human CF stimulated for 2, 4, and 8 h with 1 ng/ml of intracellularly transfected PolyIC for MDA5 and RIG-I stimulation. SiMock was used as a control. Real-time RT-PCR used to measure transcription of IL-1β, IL-6, IL-8, and TNF-α demonstrated that TLR3 partially contributed to cytosolic production of pro-inflammatory cytokines (c) but did not support direct expression of MDA5 and RIG-I (d). Means ± s.d. and values measured from one out of two independent experiments performed in duplicates are shown. Unpaired two-tailed Student t test (b), **p < 0.01 for PolyIC vs. Transf. PolyIC; two-way ANOVA with Bonferroni post hoc testing (c), **p < 0.01 for siMock vs. siTLR3