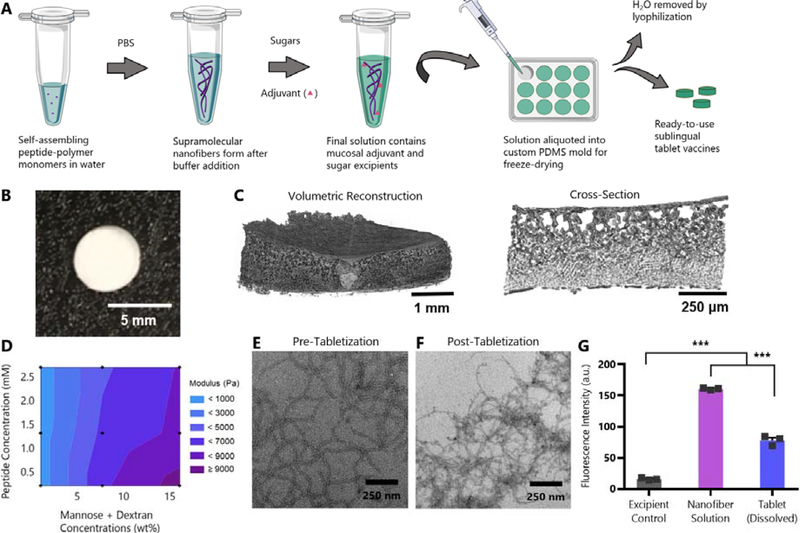
Fig. 1: SIMPL Tabletization process produces highly porous freeze-dried tablets that maintain nanofiber structure.
(A) Schematic illustrating production of SIMPL tablet vaccines. (B) Camera image of tablet. (C) Volumetric reconstruction and cross-section of tablet structure from microCT highlighting tablet porosity. (D) Contour plot showing combined effects of peptide and sugar concentration in tablets on elastic modulus. Tablets were prepared at nine combinations of peptide (OVAQ11) and sugar (dextran and mannose) concentrations (black dots on plot) and subjected to compressive testing using a micro-strain analyzer. Trehalose concentration was held constant. n=3 tablets/group, mean values shown. Individual graphs showing effects of sugar and peptide concentration individually are in Figure S2. (E-F) TEM images of PEG-Q11OVA nanofibers prepared at 2 mM (E) or a tablet dissolved at 2 mM (F), each diluted to 0.2 mM before imaging. (G) β-sheet secondary structure was assessed by thioflavin T binding of a nanofiber solution before tabletization and of an equal concentration solution of a dissolved tablet. Excipient control contained no OVAQ11. *** p < 0.001 by 1-way ANOVA with Tukey’s multiple comparisons test, n=3/group.