ABSTRACT
Background
Patients with chronic obstructive pulmonary disease (COPD) often have coagulation abnormalities. However, the factors that lead to coagulation dysfunction in acute exacerbation of COPD (AECOPD) remain insufficiently explored. This study aimed to investigate the factors affecting coagulation status in patients with COPD and their influence on thrombosis.
Methods
Data of COPD patients, including 135 cases in acute exacerbation stage and 44 cases in stable stage from Nov 2016 to Nov 2019 in our hospital, were collected. Healthy people (n = 135) were enrolled as the controls. The coagulation parameters, blood gas indexes and blood routine examination results were collected and analyzed.
Results
White blood count (WBC), neutrophil count, neutrophil percentage (N%), platelet (PLT), prothrombin time (PT), international normalized ratio (INR), fibrinogen (FIB), and activated partial thromboplastin time (APTT) increased, plasma thrombin time (TT) decreased in AECOPD group compared with the control group. In AECOPD group, PT, APTT, and FIB were positively correlated with neutrophils and C‐reaction protein levels. PT was positively correlated with PCO2 and negatively with pH. Thrombosis was observed in five acute exacerbation and three stable stage COPD patients.
Conclusions
Patients with AECOPD presented abnormal coagulation status, which was correlated to infection and hypercapnia and might be potentially the risk factor of thrombosis.
Keywords: AECOPD, coagulation, hypercapnia, infection, inflammation, thrombosis
Patients with AECOPD often have coagulation abnormalities. In this study, some factors such as infection and hypercapnia were found to be related to the coagulation abnormality and thrombosis in those patients

1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is one of the common chronic airway obstructive diseases, characterized by persistent respiratory symptoms and airflow limitation. Chronic respiratory diseases was recognised as the third leading cause of death in 2017 and COPD was the most common cause of chronic respiratory disease‐attributable deaths. 1 It is estimated that COPD will become the fourth largest cause of death in the world and potentially the direct cause of 4.4 million deaths. 2 This will place a significant burden on economic and healthcare systems throughout the world. Although the inflammation in the early stage of COPD only involves the trachea and lung, hypoxia and further acute inflammation are systemic. In acute exacerbation stage, patients developed respiratory failure often have circulatory and coagulation dysfunction, 3 , 4 which was considered the cause of thrombosis events in the patients. 3 The coagulation abnormality could cause pulmonary embolism, 5 myocardial infarction, cerebral infarction, and other thrombosis events in COPD patients. 6 These events often lead to extended hospital stay, poor prognosis, and even increased mortality.
The coagulation dysfunction and its role in COPD have been explored for years. Actually, the presence of coagulation abnormalities in smokers had been reported long ago as blood coagulation abnormalities existed in smokers and even in the passive smokers. 7 The factors involved in abnormal coagulation in COPD have also been reported. For example, acidosis could affect the coagulation status in COPD patients. 8 Respiratory bacterial infections, lipoprotein‐associated phospholipase A2, myeloperoxidase, and vascular endothelial growth factor could change the coagulation status, which could be the high risk factors of thrombotic events. 9 However, the factors lead to coagulation dysfunction in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) remain insufficient explored. Obviously, finding out the causes of coagulation dysfunction in AECOPD would help control the abnormality and secondary thrombosis risks. In this study, the coagulation status in patients with COPD was investigated and some factors such as infection and hypercapnia were found to be related to the coagulation abnormality and thrombosis in those patients.
2. MATERIALS AND METHODS
2.1. Study design
A retrospective clinical study was performed to investigate the factors correlated to abnormal coagulation status in COPD. The study was designed to collect the data of patients with COPD who were in acute exacerbation stage and hospitalized in Affiliated First Hospital, University of Science and Technology of China from November 2016 to November 2019. Inclusion criterion was a clear confirmed clinical diagnosis of COPD. The diagnosis of COPD was established by pulmonary specialists based on clinical manifestations as chronic and progressive dyspnea, cough, sputum production, and a former history of abnormal spirometry testing as FEV1/FVC < 0.70 after bronchodilator inhalation. The patients had only one diagnosis as COPD and hospitalized due to any of the following as exacerbated cough, expectoration, and dyspnea. Stable stage COPD (SCOPD) patients were those outpatients who had been diagnosed as COPD and no exacerbated symptoms. The controls were those who were in good general health with normal spirometry results, no history of cigarette smoking, had gastrointestinal polyps found in routine annual health examination, and planned to be operated. Exclusion criteria for both stages of COPD patients were a history of other pulmonary diseases, infective and inflammatory diseases, neoplastic pathologies, hematological, autoimmune, renal, gastrointestinal and hepatic diseases, and current use of anticoagulants.
The study was approved by the Ethic Committee of Affiliated First Hospital, University of Science and Technology of China (number: 2020‐P‐080), and the study was designed in accordance with the Declaration of Helsinki.
2.2. Data collection
Demographic and baseline laboratory variables including blood routine, coagulation, blood gas, inflammatory indicators were collected. In AECOPD patients, these data were collected when the patient was admitted to hospital.
2.3. Statistical analysis
Statistical analysis was performed using SPSS 22.0 (Statistical Package for the Social Sciences) statistical software. Data were presented as number of cases, mean ± standard deviation (SD), or median with interquartile range (IQR). All data were tested for normal distribution by Kolmogorov‐Smirnov test. Data for normally distributed variables were expressed as medians with IQR and tested by Mann‐Whitney test. Statistical analysis for multiple comparisons was analyzed by Kruskal‐Wallis test for non‐normally distributed variables. Pearson correlation was used to investigate the relationship between changes in coagulation and inflammatory markers. Statistical significance was defined as p < 0.05. A two‐sided p‐value < 0.05 was considered significant for all analyses.
3. RESULTS
3.1. Demographic data
A total of 135 AECOPD, 44 stable stage COPD patients, and 135 healthy controls were enrolled in this study. There were no significant differences in age, gender between AECOPD, SCOPD, and the control groups. No significant difference in disease course between AECOPD and SCOPD patients was found. The demographic characteristics of the subjects were summarized in Table 1. Several cases of thrombosis were found in AECOPD and SCOPD patients, respectively.
TABLE 1.
Demographic characteristics of the subjects
| AECOPD | SCOPD | Controls | |
|---|---|---|---|
| Number | 135 | 44 | 135 |
| Gender (M/F) | 102/33 | 34/10 | 96/39 |
| Age (years) | 72.46 ± 9.19 | 67.67 + 10.75 | 62.96 + 9.97 |
| Course of disease (years) | 12.76 ± 10.46 | 11.51 ± 11.54 | N/A |
| Cases of thrombosis | 5 | 3 | 0 |
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; SCOPD, stable chronic obstructive pulmonary disease.
3.2. Laboratory examination parameters: blood routine, CRP, and blood gas analysis
Data showed a significant difference in WBC count number and neutrophil percentage between AECOPD patients and the controls (p < 0.01). There was no significant difference in platelet count between any two of the three groups, while MPV decreased in AECOPD group (p < 0.01). No significant differences in WBC, N%, PLT were observed between the SCOPD and control group, as shown in Table 2. The data of blood gas analysis in AECOPD patients in this study were collected and retrieved finally in 89 cases. The results showed that some patients had blood gas abnormality and acidosis. Among them, 36 (40.5%) cases had an arterial blood pH lower than 7.35 and 60 (67.4%) cases had an elevated arterial carbon dioxide concentration level over 50 mmHg.
TABLE 2.
Blood routine and CRP levels of the subjects
| Normal rang | AECOPD | SCOPD | Healthy controls | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|---|
| RBC (10 ~ 12/L) |
3.50–5.50 (M) 3.8–5.10 (F) |
4.69 ± 0.71 (M) 4.41 ± 0.58 (F) |
4.58 ± 0.52 (M) 4.64 ± 0.51 (F) |
4.82 ± 0.57 (M) 4.25 ± 0.27 (F) |
0.122 (M) 0.214 (F) |
0.462 (M) 0.101 (F) |
0.031 (M) 0.033 (F) |
| Hb (g/L) |
130–175 (M) 115–150 (F) |
140.66 ± 19.38 (M) 126.92 ± 14.37 (F) |
142.26 ± 15.85 (M) 131.00 ± 12.94 (F) |
148.07 ± 10.10 (M) 130.05 ± 7.79 (F) |
0.000 (M) 0.233 (F) |
0.640 (M) 0.437 (F) |
0.020 (M) 0.882 (F) |
| PLT (10 ~ 9/L) | 125–350 | 198.93 ± 64.02 | 181.75 ± 56.19 | 199.36 ± 48.05 | 0.583 | 0.122 | 0.066 |
| MPV (fL) | 9.4–12.5 | 10.40 (9.30 11.40) | 10.40 (9.50–11.50) | 11.20 (10.20 12.20) | 0.000 | 0.668 | 0.023 |
| WBC (10 ~ 9/L) | 3.50–9.50 | 8.59 ± 5.86 | 6.42 ± 1.40 | 6.18 ± 1.36 | 0.000 | 0.003 | 0.815 |
| N (%) | 40–75 | 73.78 ± 12.15 | 58.68 ± 10.56 | 58.35 ± 7.63 | 0.000 | 0.000 | 0.084 |
| CRP (mg/L) | 0–10.0 | 21.20 (7.33 78.50) | 1.80 (1.40 2.70) | ‐ | ‐ | 0.000 | ‐ |
Data shown as mean ± SD, median (or interquartile range), P1 represents the difference between AECOPD and the controls, P2 represents AECOPD and SCOPD, P3 represents SCOPD and the controls.
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CRP, C‐reactive protein; Hb, hemoglobin; MPV, mean platelet volume; N%, Percentage of neutrophils; PLT, platelet; RBC, red blood cell; SCOPD, stable Chronic obstructive pulmonary disease; WBC, white blood cell.
3.3. Blood coagulation parameters
Prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB), and international normalized ratio (INR) in patients with AECOPD elevated significantly, and thrombin time (TT) was lower compared with the controls (16.80 vs 17.60, p < 0.05). Compared with stable COPD group, PT, APTT, FIB, INR, and other indicators in the AECOPD group were significantly higher and the differences were statistically significant. There was no significant difference in FIB and APTT between the stable stage COPD and control group. See Table 3. In addition, 48.9% (66/135) AECOPD patients had abnormal increased D‐dimer levels, while 27.8% (5/18) stable stage COPD cases were abnormal in D‐dimer levels. Abnormal D‐dimer levels were more often seen in AECOPD than in the stable COPD patients (p < 0.05).
TABLE 3.
Comparison of coagulation parameters between AECOPD, SCOPD patients, and the controls
| Normal rang | AECOPD | SCOPD | Controls | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|---|
| TT (s) | 11–21 | 16.80 (15.90 17.90) | 16.75 (15.63 18.18) | 17.60 (16.70 18.80) | 0.000 | 0.816 | 0.007 |
| PT (s) | 10.50–16.00 | 12.70 (11.70 13.70) | 11.90 (10.33 12.98) | 10.70 (10.20 11.90) | 0.000 | 0.002 | 0.014 |
| APTT (s) | 20–40 | 35.60 (31.50 39.70) | 33.05 (28.38 36.33) | 28.70 (25.00 34.70) | 0.000 | 0.015 | 0.057 |
| FIB (g/L) | 2.0–4.6 | 3.93 (3.06 5.12) | 2.94 (2.53 3.61) | 2.69 (2.28 3.13) | 0.000 | 0.000 | 0.154 |
| INR | 0.9–1.1 | 0.98 (0.91 1.11) | 0.93 (0.87 1.02) | 0.89 (0.85 0.93) | 0.000 | 0.007 | 0.032 |
| D‐dimer (mg/L) | 0–0.5 | 0.56 (0.30 1.07) | 0.38 (0.21 0.57) | ‐ | ‐ | 0.034 | ‐ |
Data shown as median (interquartile range), P1 represents the difference between AECOPD and the controls, P2 represents AECOPD and SCOPD, P3 represents SCOPD and the controls.
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; APTT, activated partial thromboplastin time; FIB, fibrinogen; INR, international normalized ratio; PT, prothrombin time; SCOPD, stable Chronic obstructive pulmonary disease; TT, plasma thrombin time.
3.4. Factors influencing coagulation status
The results of cross‐correlation analysis between various factors and coagulation indexes in AECOPD patients are showed as Figure 1. APTT, PT, and FIB levels were positive correlated with blood neutrophil counts. Among which, PT (rs = 0.317, p = 0.000) and FIB (rs = 0.234, p = 0.006) were statistically significantly. Those coagulation parameters were also positively correlated with serum CRP levels statistical significantly, as suggested by the correlation coefficients for PT (rs = 0.256), APTT (rs = 0.245), FIB (rs = 0.611). In addition, results showed that D‐dimer level was positively correlated with serum CRP levels. In 38 patients with abnormal blood gas analysis indexes, PT level was negatively correlated with pH (rs = 0.259), positively correlated with PaCO2 levels (rs = 0.301), indicating abnormal coagulation status was correlated to hypercapnia and acidosis. See Figure 1.
Figure 1.
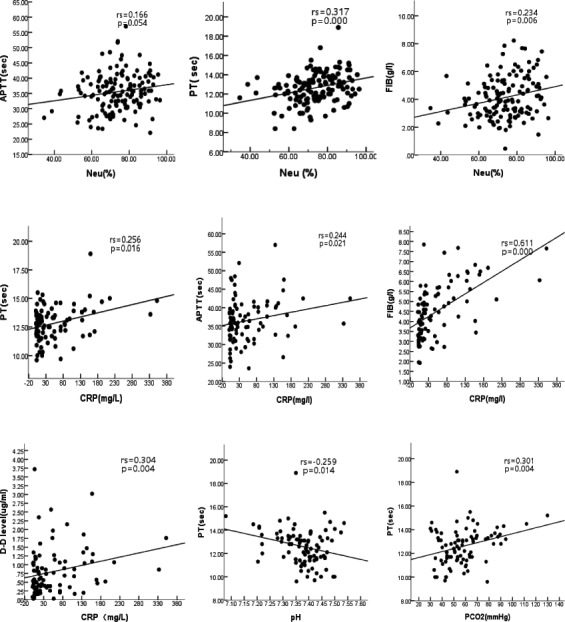
Correlation between coagulation parameters and inflammatory markers in AECOPD patients. PT, APTT, and FIB were positive correlated with blood neutrophil counts and CRP levels. D‐dimer level was positively correlated with serum CRP levels. PT level was negatively correlated with pH (rs = 0.259) and positively correlated with PaCO2 levels
4. DISCUSSION
Patients with COPD often have coagulation abnormalities. Compared with non‐COPD patients, the risk of thrombosis 10 and cardiovascular events in COPD patients is higher, particularly following an acute exacerbation. 11 In this study, the coagulation dysfunctions observed in COPD patients were found correlated with infection and hypercapnia, and potentially related to the lower limb thrombosis.
The deterioration of CODP is usually triggered by respiratory infection with bacteria or virus respiratory infections. In this study, an elevation of neutrophil number was observed compared with the stable stage COPD patients, indicating a bacterial infection existed in the AECOPD patients, see Table 2. In fact, those patients were mostly hospitalized after a confirmed respiratory infection with symptoms as exacerbated cough and spitting purulent sputum and a history of failed clinic treatments. In addition to neutrophils, CRP is also often used to characterize the systemic inflammation. 12 In the AECOPD group, CRP level increased remarkably compared with that in the stable stage patients. The changes of neutrophils and CRP suggested that the existed systemic inflammation mainly caused by the infection when the disease exacerbated. The acute exacerbation of COPD often occurs after respiratory tract infection, and the infection could increase various inflammatory mediators, cytokines, cells, and antibodies. These changes could increase the blood viscosity and affect the blood coagulation. 12 , 13
There are a wide range of interactions between coagulation and inflammations, 10 , 13 , 14 and the activation of one system may enhance the activation of the other. Coagulation disorder during systemic inflammation is an imbalance or dysfunction of tissue factor‐mediated thrombin production and normal physiological anticoagulant mechanisms. 15 When infection occurs, tissue factor rapidly increases in response to inflammatory cytokines, infection factors, free radicals, or other harmful stimuli. Then, activation of coagulation factors triggers the coagulation. 16 These could partially explain the coagulation disturbances in AECOPD patients as they often have an enhanced systemic inflammation when in acute exacerbation stage.
Respiratory failure is a common complication of AECOPD and often leads to extend hospital stay and poor prognosis. Some patients could have severe hypercapnia in the late acute exacerbation stage. In this investigation, 60 (67.4%) patients had hypercapnia with arterial carbon dioxide concentration level over 50 mmHg and 36 (40.5%) cases had acidosis. The high concentration of blood carbon dioxide and acidosis can cause dysfunction of coagulation factors 8 and blood endothelial cell damage, as reported by other researchers. 6
Coagulation dysfunction was rather common in AECOPD patients. 13 , 17 In this observation, PT and APTT were significantly prolonged and FIB was evidently elevated in the AECOPD patients (Table 3). The prolongation of the coagulation parameters (such as APTT and PT) might be caused by the consumption of clotting factors, 18 , 19 indicating that coagulation dysfunction in AECOPD patients is a complex process.
D‐dimer is an indicator of thrombosis 3 and suggested to be a prognostic biomarker for mortality in AECOPD. 20 In this study, a higher percentage of AECOPD patients had elevated blood D‐dimer level than the stable patients (48.9%, 66/135 vs 27.8%, 5/18 cases, p < 0.05), implying a thrombosis possibility in COPD especially the acute exacerbation stage patients.
The correlation analysis showed that several coagulation parameters were positively correlated with blood neutrophils number, CRP level, and arterial blood carbon dioxide concentration. D‐dimer level was correlated with serum CRP level. These results indicated a correlation between coagulation dysfunction and infection‐related inflammation and hypercapnia. As five AECOPD patients and three SCOPD patients occurred low limb thrombosis, it was reasonable to deduce that coagulation dysfunction in COPD patients was related to infection, hypercapnia, and acid‐base disturbance, and the dysfunction could be an important risk factor for the occurrence of thrombosis.
Some limitations existed in this study. Firstly, the exacerbation time before hospital admission of patients was not the same and the difference might affect coagulation parameter results. Secondly, each patient was given oxygen therapy after admission. The oxygen partial pressure data cannot be evaluated; therefore, the relationship between hypoxia and coagulation dysfunction cannot be analyzed. Thirdly, this was a retrospective study, and the number of enrolled subjects was limited. The controls were not absolute healthy people as they were found with polyps in routine physical examination and planned to be operated, although no symptoms and signs presented. Finally, the whole data of patients in hospital and later after discharge presenting the dynamic changes of coagulation status and occurrence of thrombosis could not be obtained. A more extensive study including more patients and more parameters is obviously needed.
5. CONCLUSION
In summary, this study revealed that coagulation dysfunction existed in AECOPD patients. The abnormal coagulation was correlated to infection and hypercapnia and might be the main cause of thrombosis in the patients. To understand the cause of coagulation abnormality in COPD patients and take proper measurers might be of help in managing the disease.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENT
This work was supported by the Science and Technology Foundation of Anhui Province, China (1704f0804007).
Contributor Information
Xuqin Jiang, Email: xiaodongmei@ustc.edu.cn, Email: xiaodongmei@ustc.edu.cn.
Xiaodong Mei, Email: xiaodongmei@ustc.edu.cn, Email: xiaodongmei@ustc.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. GBD Chronic Respiratory Disease Collaborators . Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all‐cause and cause‐specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. The Lancet. 2018;392(10159):2052‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pang H, Wang L, Liu J, et al. The prevalence and risk factors of venous thromboembolism in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2018;12(11):2573‐2580. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Z, Wang J, Guo M, et al. Air quality improvement during 2010 Asian games on blood coagulability in COPD patients. Environ Sci Pollut Res Int. 2016;23(7):6631‐6638. [DOI] [PubMed] [Google Scholar]
- 5. De‐Miguel‐Diez J, Albaladejo‐Vicente R, Jiménez‐García R. et al. The effect of COPD on the incidence and mortality of hospitalized patients with pulmonary embolism: a nationwide population‐based study (2016–2018). Eur J Intern Med. 2021;84:18‐23. [DOI] [PubMed] [Google Scholar]
- 6. Malerba M, Nardin M, Radaeli A, Montuschi P, Carpagnano GE, Clini E. The potential role of endothelial dysfunction and platelet activation in the development of thrombotic risk in COPD patients. Expert Rev Hematol. 2017;10(9):821‐832. [DOI] [PubMed] [Google Scholar]
- 7. Tapson VF. The role of smoking in coagulation and thromboembolism in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 8. White H, Bird R, Sosnowski K, Jones M. An in vitro analysis of the effect of acidosis on coagulation in chronic disease states ‐ a thromboelastograph study. Clin Med. 2016;16(3):230‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seri A, Marta DS, Madalan A, Popescu M, Tiglea AI, Moldoveanu E. Lipoprotein‐associated phospholipase A2, myeloperoxidase and vascular endothelial growth factor ‐ predictors of high vascular risk in respiratory bacterial infections. Journal of medicine and life. 2016;9(4):429‐433. [PMC free article] [PubMed] [Google Scholar]
- 10. Marongiu F, Mameli A, Grandone E, Barcellona D. Pulmonary thrombosis: a clinical pathological entity distinct from pulmonary embolism? Semin Thromb Hemost. 2019;45(8):778‐783. [DOI] [PubMed] [Google Scholar]
- 11. Børvik T, Brækkan SK, Enga K, et al. COPD and risk of venous thromboembolism and mortality in a general population. Eur Respir J. 2016;47(2):473‐481. [DOI] [PubMed] [Google Scholar]
- 12. van der Vorm LN, Li L, Huskens D, et al. Acute exacerbations of COPD are associated with a prothrombotic state through platelet‐monocyte complexes, endothelial activation and increased thrombin generation. Respir Med. 2020;171:106094. [DOI] [PubMed] [Google Scholar]
- 13. Husebø GR, Gabazza EC, D'Alessandro Gabazza C, et al. Coagulation markers as predictors for clinical events in COPD. Respirology. 2021;26(4):342‐351. [DOI] [PubMed] [Google Scholar]
- 14. MacCallum PK. Markers of hemostasis and systemic inflammation in heart disease and atherosclerosis in smokers. Proc Am Thorac Soc. 2005;2(1):34‐43. [DOI] [PubMed] [Google Scholar]
- 15. Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26‐34. [DOI] [PubMed] [Google Scholar]
- 16. Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118(9):1392‐1408. [DOI] [PubMed] [Google Scholar]
- 17. Ashoor TM, Hasseb AM, Esmat IM. Nebulized heparin and salbutamol versus salbutamol alone in acute exacerbations of chronic obstructive pulmonary disease requiring mechanical ventilation: a double‐blind randomized controlled trial. Korean J Anesthesiol. 2020;73(6):509‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu J, Pang J, Ji P, et al. Coagulation dysfunction is associated with severity of COVID‐19: a meta‐analysis. J Med Virol. 2021;93(2):962‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fruchter O, Yigla M, Kramer MR. D‐dimer as a prognostic biomarker for mortality in chronic obstructive pulmonary disease exacerbation. Am J Med Sci. 2015;349(1):29‐35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


