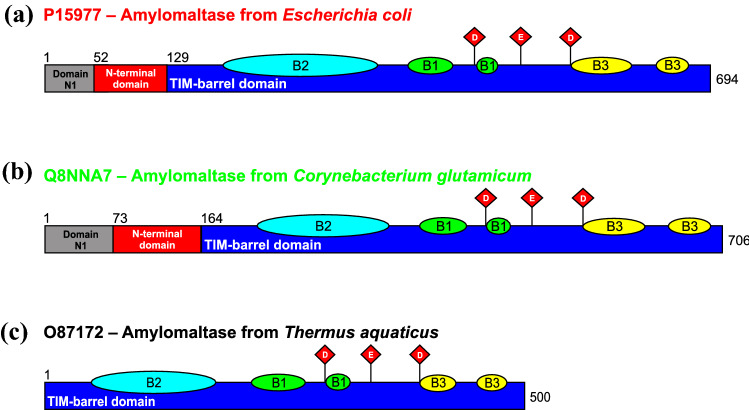
Fig. 1.
Domain arrangement of selected amylomaltases of family GH77. The enzymes from E. coli (a) and C. glutamicum (b) represent the group of bacterial amylomaltases with N-terminal extensions, a part of which, indicated as the “N-terminal domain”, constitutes a presumed CBM, i.e. a novel SBD. Individual domains or subdomains are coloured as follows: domain N1—grey; N-terminal domain (the potential novel SBD CBM family)—red; catalytic TIM-barrel domain A—blue; subdomains B1, B2 and B3—green, cyan and yellow, respectively. The residues of the catalytic triad of family GH77 (and hence of the entire clan GH-H)—aspartic acid, glutamic acid and aspartic acid, positioned on strands β4, β5 and β7, respectively, of the TIM-barrel, are shown as red diamonds. For comparison, the domain arrangement of a typical bacterial Thermus-like GH77 amylomaltase from Thermus aquaticus (c) is also shown