Fig. 6.
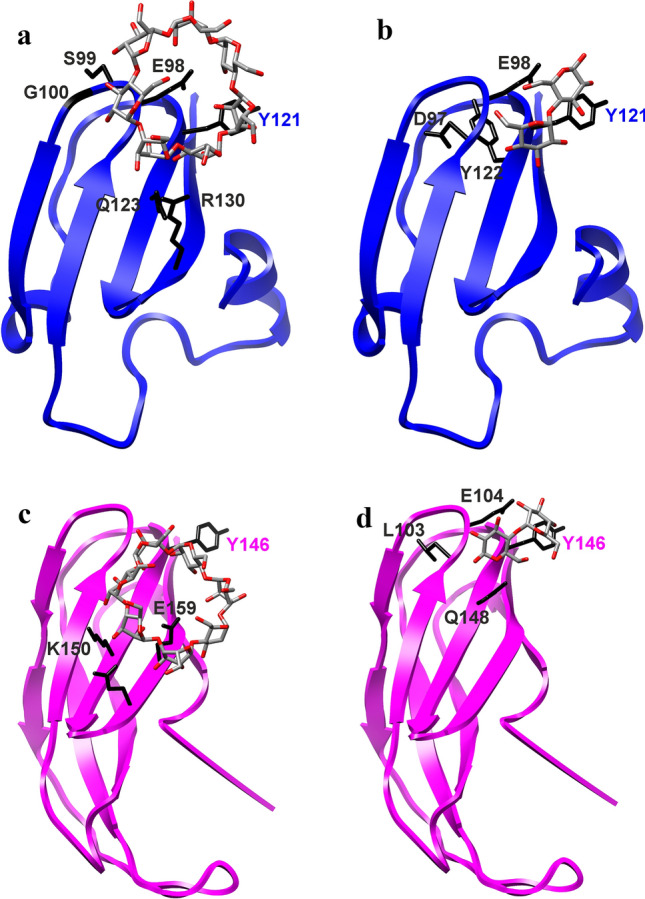
Visualisation of molecular docking experiments with modelled structures. The models of the N-terminal domain of family GH77 amylomaltases from: a, b Kushneria marisflavi (UniProt: A0A24OU528; template: 4S3R; Weiss et al. 2015) and c, d Pelotomaculum thermopropionicum (UniProt: A5D1W1; template: 5B68; Joo et al. 2016) with docked β-cyclodextrin (a, c) and maltose (b, d). Side-chains of residues potentially involved in carbohydrate binding are displayed (in black) and labelled accordingly (for details, see Table 1). The best-conserved aromatic residue, i.e. Tyr121 of the K. marisflavi amylomaltase and Tyr146 of the P. thermopropionicum counterpart (cf. Figure 2) and possibly involved in stacking interactions with glucose moieties, is also highlighted, regardless of whether it is involved or not in hydrogen bond contacts during the docking trials (Table 1)
