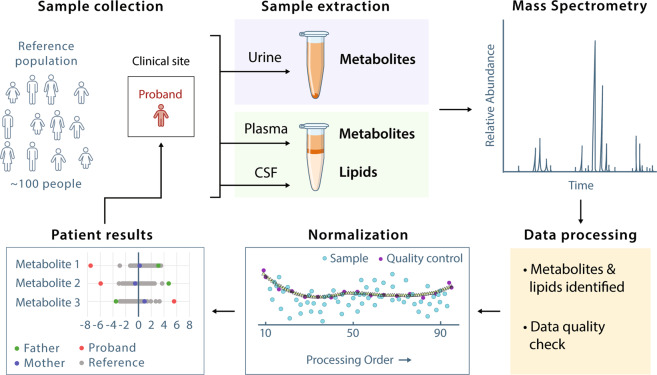
Fig. 1.
Overview of the study design. Biofluid samples were collected from probands at the UDN clinical sites and then extracted for metabolomics (urine, plasma, CSF) and lipidomics (plasma and CSF) analyses using chromatography coupled to mass spectrometry (GC-MS for metabolomics and LC-MS/MS for lipidomics). Data were pre-processed, including data quality checks, normalized, and compared against data from the reference population of healthy individuals. Metabolomics and lipidomics results in the form of Z-score, log2 fold change and p-value per metabolite and lipid of the proband (and associated family members, if applicable) were reported back to the respective UDN Clinical Site for diagnostic assistance.