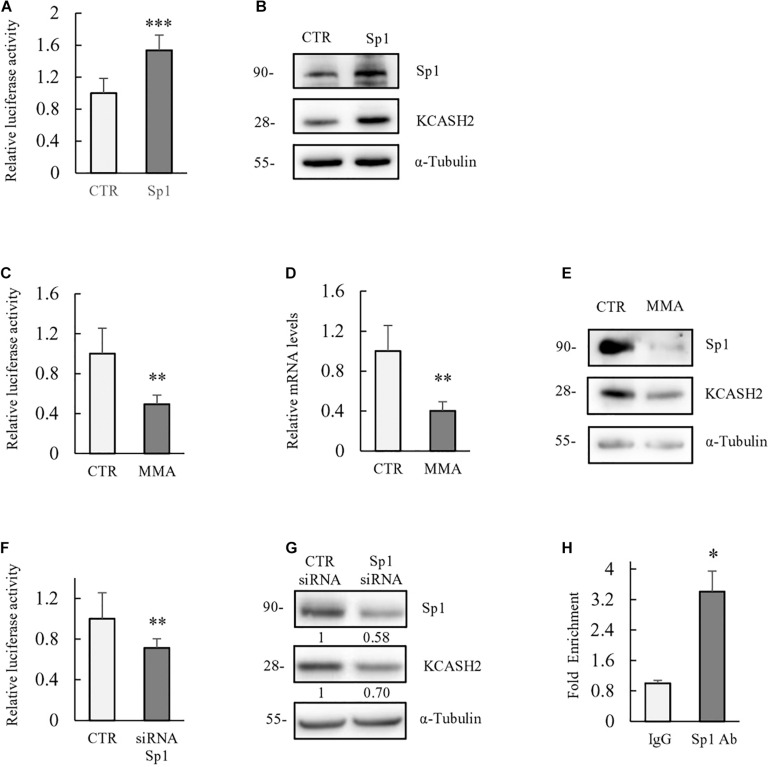
FIGURE 2.
Basal transcriptional activity of human KCASH2 promoter is driven by Sp1. (A,B) Sp1 ectopic expression increases KCASH2 promoter activity and KCASH2 protein levels. (A) HEK293T were co-transfected with the KCASH2 promoter luciferase reporter, together with control (CTR) vector or Sp1 expressing plasmid. Luciferase activity was normalized to the control. ∗∗∗p < 0.001. (B) HEK293T were transfected with empty vector or Sp1 expressing vector; 24 h later, cells were lysed and analyzed by Western blot, using Sp1 and KCASH2 antibodies. Tubulin was used as loading control. (C–G) Inhibition of Sp1 leads to a decrease in KCASH2 reporter activity, KCASH2 mRNA, and protein levels. (C) HEK293T cells were co-transfected with KCASH2-Luc reporter and treated with MMA (200 nM) for 24 h. Luciferase activity was normalized to mock treated cells (CTR). ∗∗p < 0.01. (D,E) HEK293T cells were cultured with MMA (200 nM) for 48 h. Then, cells were lysed and KCASH2 mRNA (D) and protein levels (E) were evaluated by RT-qPCR and Western Blot, respectively. KCASH2 mRNA levels were normalized to GAPDH, TBP, ß2M, and represented as fold-induction of the CTR. ∗∗p < 0.01. Western Blot was performed as above. Transfection efficiency in luciferase experiments was normalized by co-transfection of a pRL–TK–Renilla reporter. All experiments represented are the mean of three independent experiments ± standard deviation (SD). (F) Relative luciferase activity was measured in HEK293T cells transfected with scrambled siRNA (siCTR) or with Sp1 siRNA followed by KCASH2-Luc reporter and pRL–TK Renilla. Luciferase activity was normalized to the control. ∗∗p < 0.01. (G) HEK293T were transfected with siCTR or with Sp1 siRNA, 24 h later, cells were lysed and analyzed by Western Blot, using Sp1 and KCASH2 antibodies. Tubulin was used as loading control. Band intensities were analyzed using image J software and numbers below the boxes represent the relative quantification of protein levels. (H) Sp1 binds in vivo to the KCASH2 promoter sequence. Cross-linked chromatin was extracted from HEK293T cells and immunoprecipitated with a relevant control IgG or specific anti-Sp1 antibody. Immunoprecipitated chromatin samples were analyzed by qPCR using KCASH2 promoter selective primers. Relative enrichment was calculated by Delta CT analysis and expressed as fold induction of immunoprecipitated IgG negative control vs. specific anti-Sp1 antibody. ∗p < 0.05.