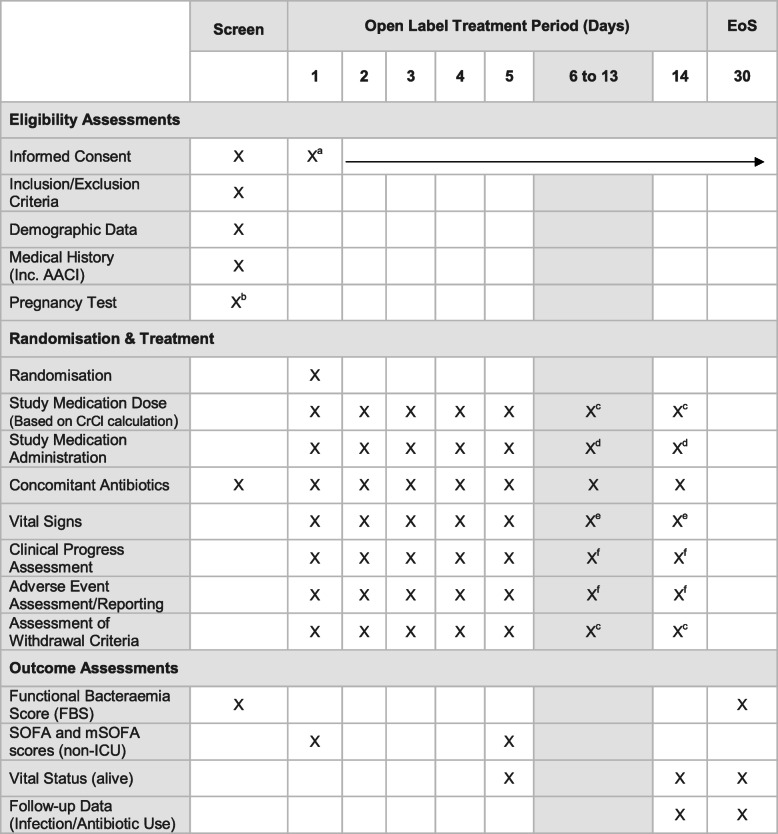
Table 3.
Study time and event schedule
aWritten informed consent at screening or day 1 prior to any study procedure. Continue to obtain verbal consent for the continuation of participation
b Females of child-bearing potential. Only required if routine clinical pregnancy test result is not available. Urine or blood test as per local practice. Where possible in unison with other routine clinical tests
cIf participant remains/remained on study treatment
dClinician decision to cease or continue study medication up to and including day 14
eOnly if the participant remains in the hospital
f If participant remains on study treatment or within 24 h of the last dose of study treatment
NB. Index blood culture and any subsequent blood cultures will be taken as part of routine clinical practice during the 30-day trial period if the participant is febrile—defined as temperature ≥ 38.0 °C
Routine clinical haematology and biochemistry results will be used to calculate SOFA, mSOFA and CrCl