Table 1.
MCL-1 targeted anti-tumor drugs in clinical trials
| Agents | Frist report time (drug discovery method) | Identifier/phase | Population | Regimen |
|---|---|---|---|---|
| Monotherapy | ||||
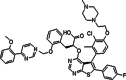
|
(MIK665) |
2016 (NMR-based fragment screen) |
NCT02979366 Phase 1 | AML, MDS | Once or twice a week (21/28-day cycle), the starting dose is 50 mg (intravenous) |
| NCT02992483 Phase 1 | MM, DLBCL | Dose finding (intravenous) | ||
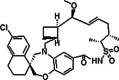
| AZD5991 |
2018 (NMR-based fragment screen and Structure-based design) |
NCT03218683 Phase 1 | AML, CLL, MDS, MM | Intravenously for 9 cycles (21-day cycle) |
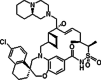
| AMG-176 |
2016 (High-throughput screening) |
NCT02675452 (Suspended)Phase 1 | MM, AML | Dose finding(intravenous) |
| AMG-397 |
2018 (-) |
(Suspended)Phase 1 |
MM, NHL, AML, DLBCL | Once a day for 2 consecutive days followed by 5 days break at a weekly interval (28-day cycle) (oral) |
| ABBV-467 | NA |
NCT04178902 Phase 1 2019 |
MM and Cancer | Dose finding (intravenous) |
| PRT1419 | NA |
NCT04543305 Phase 1 2020 |
MM, NHL, AML, MDS | Dose finding (oral) |
| Combination therapy | ||||
| S64315 + Venetoclaxv | NCT03672695 Phase 1 | AML |
S64315 once a week (intravenous) Venetoclax once a day (oral) (21-day cycle) |
|
| AZD5991 + Venetoclaxv | NCT03218683 Phase 2 | AML, MDS | Ascending oral doses of Venetoclax | |
| AMG-176 + Venetoclaxv |
(Suspended)Phase 1 |
AML, NHL/DLBCL | Two-consecutive days per week (QD2) | |
RS Richter syndrome, SLL Small lymphocytic lymphoma