FIGURE 1:
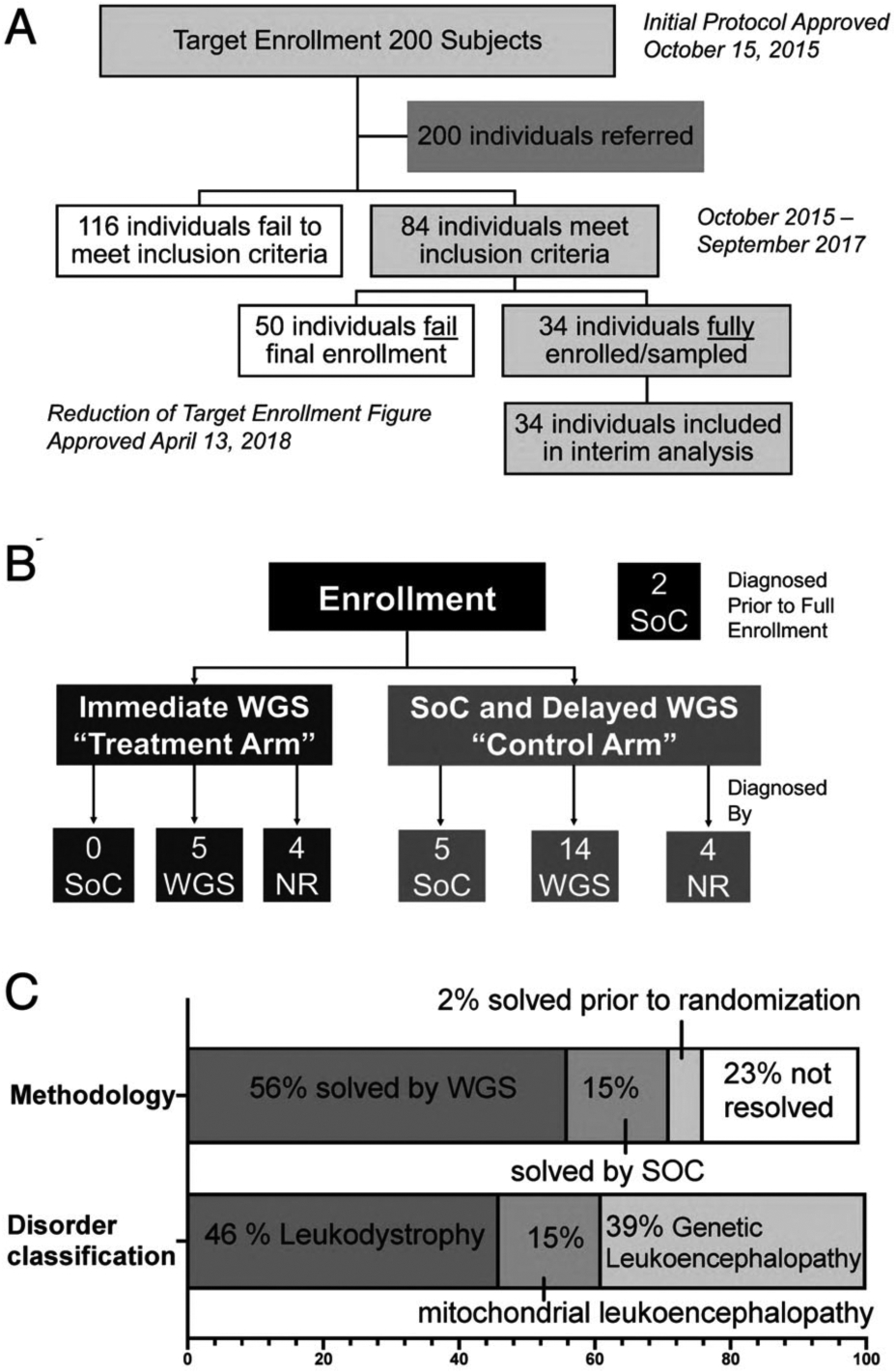
LeukoSeq trial design and principal results. (A) Overall recruitment and enrollment of the cohort examined in this study. (B) The trial employed a time-delayed crossover design with one-third of individuals assigned to the immediate genome sequencing (GS) arm and two-thirds of individuals receiving standard of care (SoC) for 4 months followed by GS. Of the 9 individuals assigned to immediate- GS, none received a diagnosis during the study period using SoC, 5 received a diagnosis using GS, and 4 did not achieve a diagnosis. Of the 23 individuals undergoing SoC with delayed-GS, only 5 individuals achieved a diagnosis with SoC approaches. Fourteen individuals achieved a diagnosis using GS, and 4 individuals did not achieve a diagnosis. Two patients noted in the black box at the top right received a diagnosis prior to randomization. (C) Distribution of cases solved by modality (SoC or GS) and broad class of white matter disorder. NR = not resolved; WGS = whole genome sequencing.
