Fig. 3. Pharmacokinetics of M3mP6 HLPN.
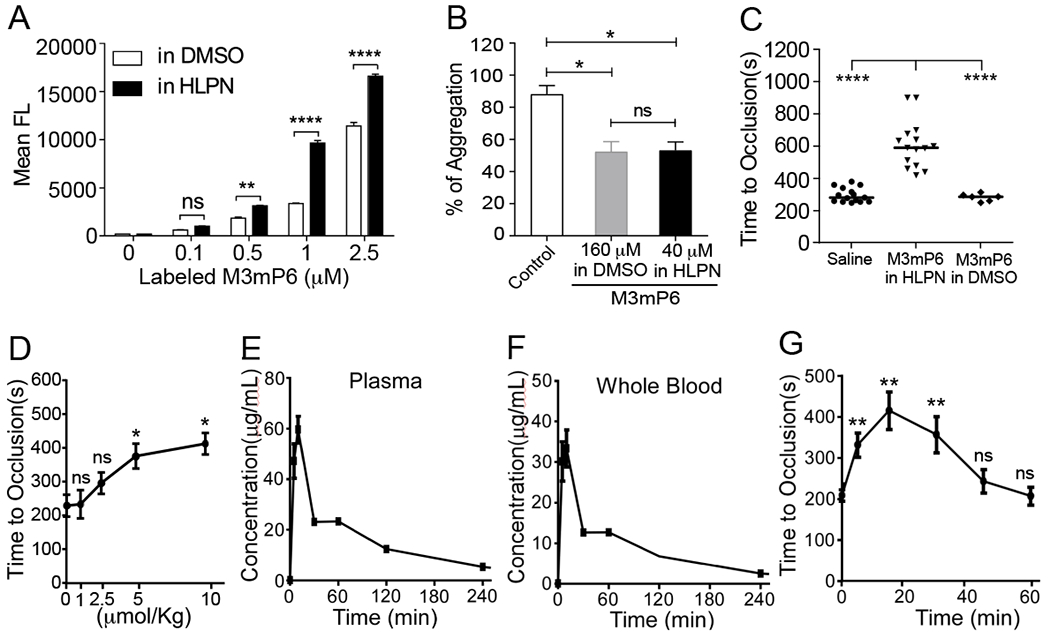
(A) Flow cytometry comparison of the uptake of fluorescence-labeled M3mP6 dissolved in DMSO with that in HLPN into mouse platelets (n=3). (B) Comparable effect of 40 μM M3mP6 in HLPN with 160 μM DMSO-solubilized M3mP6 on human platelet aggregation (n=3). (C) Effects of M3mP6 HLPN (10 μmol/kg) on FeCl3-induced carotid artery thrombosis (n=15), as compared with 10 μmol/kg DMSO-solubilized M3mP6 (n=6) and with saline control (n=15). (D) Dose response of M3mP6 HLPN in inhibiting FeCl3-induced carotid artery occlusive thrombosis following retro-orbital injection 15 minutes before procedure (n=3). (E) Pharmacokinetic study on plasma concentrations of M3mP6 HLPN following retro-orbital injection (5 μmol/kg). (F) Pharmacokinetic study on whole blood levels concentrations of M3mP6 HLPN following retro-orbital injection (5 μmol/kg). (G) Kinetics of anti-thrombotic effect of 5 μmol/kg M3mP6 HLPN (retro-orbital injection) on FeCl3-induced carotid artery occlusive thrombosis (n=3~5). **P<0.01, ****P<0.0001, n.s., not significant, one-way ANOVA with multiple comparisons in (A) and (B); Mann-Whitney test in (C); unpaired t-test in (D) and (G); data presented as means ± SEM.
