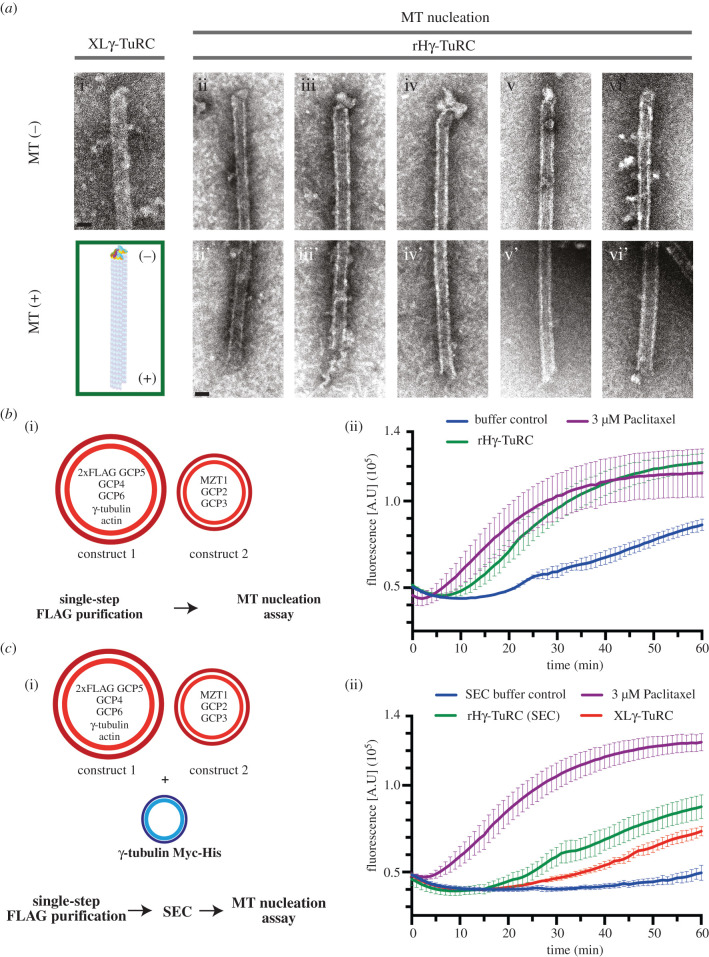
Figure 4.
The recombinant human γ-TuRC has microtubule nucleation and minus end capping activity. (a) Representative negative stain EM images from microtubules nucleated by native X. laevis γ-TuRC; (i; XLγ-TuRC) or the recombinant human γ-TuRC (ii-vi, rHγ-TuRC). For microtubules nucleated by the recombinant human γ-TuRC, the capped microtubule minus ends (ii–vi; MT-) and the flared or sheet microtubule plus ends (ii'–vi'; +MT) are shown. A schematic representation of a γ-TuRC capped microtubule is shown in the green box. Scale bars, 25 nm. (b,c) Tubulin polymerization assay where the increase in fluorescence intensity over time represents αβ-tubulin polymerization into microtubules. Shown are error bars for the standard deviation of the mean of three (b) and four (c) technical replicates. (b) The recombinant human γ-TuRC (construct 1 and construct 2 in figure 1, rHγ-TuRC) was analysed together with elution buffer without recombinant γ-TuRC as negative control, and 3 µM Paclitaxel as positive control. (c) Recombinant human γ-TuRC (construct 1, construct 2 and C-terminal Myc-His6 tagged γ-tubulin) was purified by FLAG affinity purification and subsequent SEC (figure 3b i). The γ-tubulin content of ‘γ-TuRC peak’ fraction (rHγ-TuRC SEC) and native X. laevis γ-TuRC (XLγ-TuRC) was determined by immunoblotting. Samples were diluted in SEC buffer to equal γ-tubulin concentrations and were then used for the microtubule nucleation assay with SEC buffer as a negative control and 3 µM Paclitaxel as positive control.