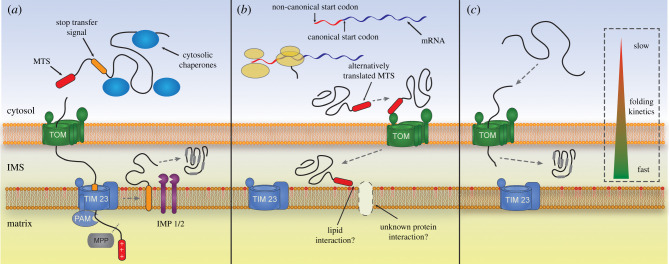
Figure 3.
Alternative protein import pathways to the mitochondrial IMS. Nuclear-encoded, Mia40-independent proteins destined for the IMS are imported and retained in a number of different ways. (a) Proteins using the stop-transfer pathway (e.g. cytochrome b2) contain a bipartite signal composed of a positively charged mitochondrial targeting signal (MTS) at the proteins N-terminus followed by a hydrophobic segment. The MTS is targeted through the translocon of the outer membrane (TOM) and the translocon of the inner membrane (TIM23) into the matrix via the presequence translocase-associated motor (PAM). Further translocation to the matrix is blocked when the stop-transfer hydrophobic signal enters TIM23 and causes translocational arrest followed by lateral diffusion of this segment into the inner membrane. The MTS is cleaved by the mitochondrial processing peptidase (MPP) and a mature IMS protein is released via a second cleavage event mediated by IMS proteases such as IMP1/2. (b) Under stress conditions glutathione peroxidase 3 (Gpx3/Hyr1) is alternatively translated from a non-AUG start codon producing an extended protein containing a signal resembling an MTS. This extension enhances the mitochondrial localization of Gpx3 most likely by targeting Gpx3 via TOM, however whether this MTS interacts with TIM23 of some other inner membrane component is currently unknown. (c) Some slowly folding proteins in the cytosol can be transported into the IMS in an unfolded state, probably through the TOM, where increased folding kinetics leads to their retention in the IMS (e.g. Adk1 and Ccs1). Little is known however about the actual import pathway of this subset of IMS proteins.