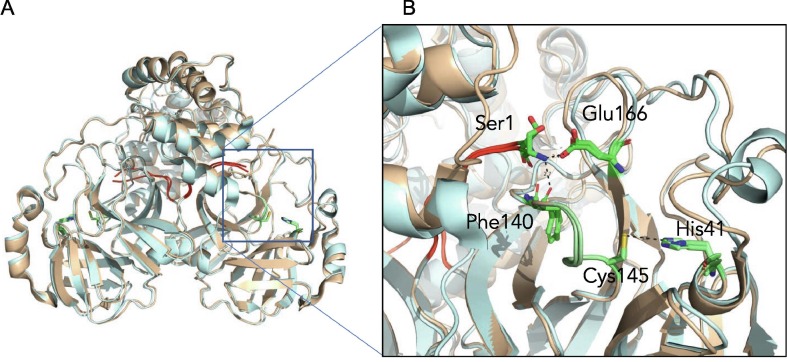
Figure 7.
The N terminus of one protomer interacts with the active site region of the other protomer. (A) Comparison of SARS-CoV Mpro (wheat) and SARS-CoV-2 Mpro (cyan) structures reveals the N terminus of each protomer (red) participates in domain swapping in the other protomer. (B) Hydrogen bonding with the N-terminal Ser1 occurs with the side chain of Glu166 and backbone oxygen of Phe140. This influences the region adjacent to the catalytic residues Cys145 and His41, and the oxyanion hole, colored in green.