Abstract
The semi-allogeneic fetus develops in a uniquely immune tolerant environment within the uterus. For successful pregnancy, both the innate and adaptive immune systems must favor acceptance of the fetal allograft. Macrophages are the second most abundant immune cells after natural killer (NK) cells in the decidua. In coordination with decidual NK cells and dendritic cells, macrophages aid in implantation, vascular remodeling, placental development, immune tolerance to placental cells, and maintenance of tissue homeostasis at the maternal-fetal interface. Decidual macrophages show the classical activated (M1) and alternatively activated (M2) phenotypes under the influence of the local milieu of growth factors and cytokines, and appropriate temporal regulation of the M1/M2 switch is vital for successful pregnancy. Disturbances in the mechanisms that control the M1/M2 balance and associated functions during pregnancy can trigger a spectrum of pregnancy complications ranging from preeclampsia and fetal growth restriction to preterm delivery. This review addresses various mechanisms of tolerance, focusing on the basic biology of macrophages, their plasticity and polarization, and their protective roles at the immune-privileged maternal-fetal interface, including direct and indirect roles in promoting fetomaternal immune tolerance.
Keywords: macrophages, maternal-fetal interface, immune tolerance, pregnancy, decidua, placenta
1. INTRODUCTION
Despite its allogeneic nature, the fetal allograft is not normally rejected by the potentially hostile maternal immune system. The mystery of immunological acceptance of the fetal allograft has been the focus of many studies. There are various local and systemic mechanisms that induce maternal tolerance. These include production of transforming growth factor β1 (TGF-β1) and interleukin-10 (IL-10) by the regulatory T (Treg) subset of CD4+/CD25 cells, secretion of prolactin, gonadotropin and progesterone by both fetal and endometrial cells, and expression of high levels of complement regulatory proteins by fetal cells and expression of inhibitory members of the B7 and the tumor necrosis factor (TNF) family of ligands [1–3]. Fetal cells also produce immunosuppressive cytokines, chemokines, and prostaglandins, which dampen T lymphocyte proliferation, and secrete the immunosuppressive hormone progesterone.
Importantly, trophoblast cells regulate the expression of classical major histocompatibility complex (MHC-Ia) and non-classical (MHC-Ib) proteins. Conceptus-derived villous trophoblast cells do not express highly polymorphic MHC-Ia and MHC class II molecules. However, it has been shown in many species (for example, human, mouse, rhesus macaque, baboon, cattle, etc.) that trophoblast cells upregulate monomorphic and oligomorphic MHC-Ib proteins in trophoblast cells. The human MHC-Ib molecule, human leukocyte antigen G (HLA-G) provides inhibitory signals to NK cells and other lymphocytes, macrophages, and monocytes by interacting with various inhibitory receptors expressed by leukocytes to induce immunosuppression and fetal survival [4,5]. Inhibitory leukocyte immunoglobulin-like receptor B (LILRB) expression is enriched in myeloid cell populations and negatively regulates myeloid cell activation.
LILRB4 activation in monocytes and macrophages attenuates Ca2+ influx resulting from CD11b, HLA-DR, and Fcγ-RIII acute activation in human myeloid cell lines [6] and increases IL-10 while reducing IL-8 [7], supporting a role for LILRBs in regulating innate immune inflammatory responses.
Macrophages serve as crucial players in immunological tolerance at the maternal fetal interface. In addition to their primary role as major antigen presenting cells involved in both innate and adaptive immunity in the decidua, they are actively involved in trophoblast invasion, tissue and vascular remodeling during early pregnancy, and phagocytosis of pathogens, debris, and dead cells [8]. They are usually divided into pro-inflammatory (M1) and anti-inflammatory (M2) types, depending upon the tissue microenvironment. The local cytokine milieu and signals can determine the polarity of macrophages.
In this review we discuss the roles of decidual macrophages, and their plasticity and polarization in the context of pregnancy and how these contribute to fetomaternal immunological tolerance.
2. INNATE IMMUNE CELLS AT THE MATERNAL-FETAL INTERFACE
Antigen-presenting cells are likely to be important players in the mediation of immune tolerance in the decidua. The study of decidual dendritic cells (dDCs) has been difficult, not only because isolation of decidual cells, including dDCs, can be technically demanding, but also because phenotypic definition of DCs is controversial, as there is no single specific marker for DCs. Macrophages are specialized phagocytic cells of the innate immune system and they are present in every organ of the body in one form or another. Macrophages originate from bone marrow and enter and circulate in peripheral blood [9,10]. Circulating monocytes, both for homeostasis and during inflammation, migrate into tissues and differentiate into macrophages when exposed to local growth factors, pro-inflammatory cytokines, and microbial products. Macrophages, like DCs, are part of the monocyte-macrophage system consisting of committed bone marrow precursors, peripheral blood monocytes and DCs, and tissue macrophages and DCs [11].
2.1. Placentation and cells in the decidua
During pregnancy, the uterus undergoes enormous transformation, starting at conception and continuing throughout gestation, labor, and birth. The blastocyst invades the mother’s uterine endometrium, endometrial stromal cells undergo decidualization and create an environment favorable for trophoblast invasion [12]. Based on the apposition pattern and degree of contact of trophoblast cells with the uterine lining, mammals have three types of placentation: epitheliochorial, endotheliochorial, and hemochorial [13]. Humans and mice have hemochorial placentas where the fetal membrane is in direct contact with the maternal tissue and blood. For successful pregnancy, a finely orchestrated balance of immune cell subsets and the fetal interface is required for appropriate recognition and tolerance of the fetal allograft [14].
About 40% of the decidua is composed of maternal leukocytes [15]. In the first trimester, the predominant leukocytes in the decidua basalis (the site where endometrium interacts with trophoblast cells) are decidual natural killer cells [dNK] (~70%) followed by decidual macrophages, dendritic cells, and T cells [14,16,17]. Almost 20-30% of leukocytes are decidual macrophages, which serve important functions, such as the promotion of tolerance to the semi-allogeneic fetus, trophoblast invasion, and tissue and vascular remodeling [18–21]. These macrophages are recruited to the decidua in response to growth factors and cytokines, such as colony-stimulating factor −1 (CSF-1), and granulocyte macrophage-colony stimulating factor (GM-CSF) secreted by trophoblasts [22]. Recently, vascular endothelial growth factor (VEGF) produced by macrophages, decidualized endometrial cells, and trophoblasts has also been implicated in the regulation of the migration and polarity of macrophages in the decidua and other tissues [23–28]. VEGF, although essential for normal embryonic development, could also be a trigger for elevated placental soluble FLT1 (sFLT1) leading to the preeclampsia symptoms as shown in mice models. Placental sFLT1 has a protective role for the placenta and the fetus through its sequestration of maternal free VEGF [29,30].
2.2. Macrophage markers
Macrophages display distinct cell surface markers, such as CD14, CD68, and human leukocyte antigens, such as HLA-DR [31]. The phenotype of decidual macrophages is believed to be influenced by trophoblasts which secrete chemokines and cytokines. Human decidual macrophages are CD163+CD206+DC-SIGN+ and predominantly express IL-10, CCL2 and CCL18 [32–35]. Compared to the peripheral blood monocytes, decidual macrophages express lower levels of the co-stimulatory molecule CD86 [31]. Lower expression of CD86, coupled with expression of indoleamine 2,3 dioxygenase (IDO), enables macrophages to exert an immunosuppressive effect on T cells [31].
3. THE ROLE OF MACROPHAGES IN PREGNANCY
Establishment and maintenance of an immunologically naive environment in the uterus to protect the semi-allogeneic fetus during pregnancy is a challenge for the immune system. Uterine mucosal and decidual macrophages play myriad roles during pregnancy. They participate in remodeling the decidua and spiral arteries, a process that is triggered by growth factors, angiogenic factors, and cytokines produced by decidual NK cells, the most abundant immune cells during the first half of pregnancy. This promotes trophoblast invasion into the endometrium and thus increases the availability of maternal blood to the growing fetus [36,37]. During vascular remodeling, spiral arteries lose smooth muscle cells and endothelial cells in response to Fas ligand, TNF-α, and matrix metalloproteinases (MMP)-2 and −9 secreted by the invading trophoblast cells, leading to high-volume, low-resistance utero-placental maternal blood flow [38,39]. Apoptosis of endothelial smooth muscle cells is induced by Fas ligand secreted by endothelial cells and macrophages/monocytes [40,41]. Decidual macrophages also produce VEGF and MMP-9 in vitro, suggesting roles in angiogenesis and tissue remodeling [35,42,43]. Disruption or impairment of vascular remodeling results in pregnancy pathologies, such as preeclampsia and fetal growth restriction [44].
Decidual macrophages are major phagocytes which phagocytose and clean up apoptotic trophoblasts, cell debris, and foreign pathogens, thus preventing proinflammatory reactions at the maternal-fetal interface [45]. Decidual macrophages are plastic cells that possess pattern recognition receptors (PRRs) on their surfaces. In the presence of pathogens, these PRRs, such as CD163 (hemoglobin scavenger receptor), CD206 (mannose receptor), and CD209 (DC-SIGN), promote polarization of these plastic cells toward the M1 phenotype, suggesting they play a canonical antimicrobial role in protecting the fetus against infections [46].
Infection by diverse pathogens induces recruitment of circulating monocytes and macrophages to the sites of inflammation, which involves the pathogen-associated molecular patterns (PAMPs) released from invading pathogens and damage-associated molecular patterns (DAMPs) released from damaged or dead cells. Furthermore, tissue resident memory T cells activate and secrete inflammatory cytokines and chemokines, which also triggers macrophage recruitment [47,48]. Other factors that contribute to the development and activation of the phenotypic profile of macrophages in the pregnant uterus of mice and humans are IFN-γ, produced by uterine NK cells, and CSF-1 (M-CSF), secreted by uterine epithelial and stromal cells [49,50].
Decidual macrophages, NK cells, and T cells: a crosstalk
Endometrial NK cells are differentiated into activated decidual NK cells through the secretion of IL-15 by decidual macrophages [51]. Activated dNK cells induce expression of Tregs which suppress maternal alloreactive T cells. Interaction between decidual macrophages and NK cells also leads to secretion of interferon-gamma (IFN-γ) by dNK cells, which, in turn, induces IDO in macrophages, further suppressing T cell activation and inducing differentiation of Tregs [52]. Decidual macrophages interact with PD-1 on T cells through B7-H1 and B7-DC (B7 family costimulatory molecules) and negatively modulate the activity of T cells contributing to dampened immune responses during early human pregnancy [53]. Furthermore, B7-1 and −2 on macrophages also interact with CTLA-4 on Tregs leading to immunosuppressive functions and upregulating IDO in macrophages. Therefore, dNK cell-macrophage, and T cell-macrophage interactions contribute to maternal-fetal immune tolerance [54] (Figure 1).
Figure 1: Crosstalk between decidual macrophages, decidual NK, and T cells.
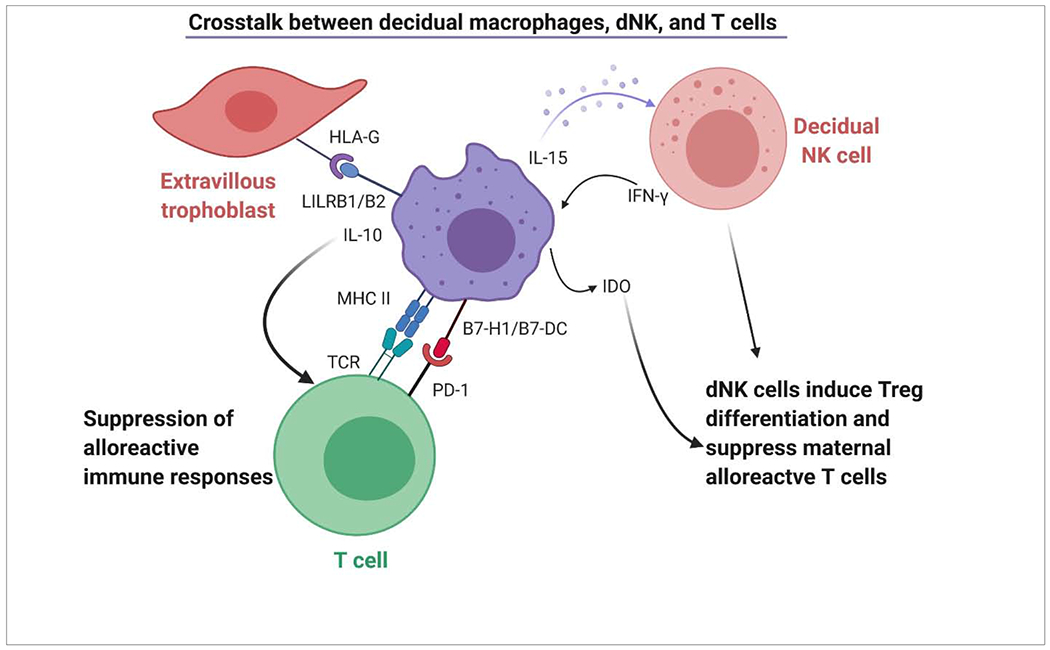
Decidual macrophages secrete IL-15 inducing differentiation of endometrial NK cells into active decidual NK cells. dNK cells suppress maternal alloreactive immune responses through induction of Tregs. Macrophages by effect of dNK-secreted IFN-γ produce IDO which further suppress T cell activation and induces differentiation of Tregs. Decidual macrophages interact with PD-1 on T cells through B7-H1 and B7-DC (B7 family costimulatory molecules) and negatively modulate the activity of T cells.
4. MACROPHAGE POLARIZATION AND PREGNANCY AS A PARADIGM
Macrophages possess remarkable plasticity and are capable of switching from one phenotype to another [55–57]. Macrophages are functionally classified as classically activated (M1) and alternatively activated (M2) phenotypes, depending on their cytokine production [58,59]. The M1 phenotype is proinflammatory and induced by pathogen (lipopolysaccharide) exposure and tissue damage through IFN-γ and tumor necrosis factor (TNF). In contrast, M2 macrophages are regulatory/homeostatic and anti-inflammatory in nature and are induced by Th2 cytokines such as interleukin-4 (IL-4) and interleukin-13 (IL-13), and anti-inflammatory IL-10, as well as apoptotic cells (ACs), and macrophage colony-stimulating factor (MCSF or CSF-1) [14,55,59]. Macrophage subsets predominantly producing inducible nitric oxide synthase (iNOS) over arginase are of the M1 phenotype, and those producing arginase over iNOS are of the M2 phenotype. M2 subsets enhance Treg accumulation and are subgrouped into M2a, M2b, M2c, and M2d based on the applied stimuli and the resultant transcriptional changes [60]. M2a subsets are activated by IL-4 and IL-13, resulting in increased expression of IL-10, TGF-β, CCL17, CCL18, and CCL22. These macrophages enhance endocytic activity and promote cell growth, and tissue repair. M2b macrophages are induced by immune complexes, Toll-like receptor (TLR) ligands, and IL-1β and secrete both pro- and anti-inflammatory cytokines and chemokines. M2c macrophages are referred to as inactivated macrophages and are activated by IL-10, TGF-β, and glucocorticoids. These cells are capable of clearing early ACs more efficiently than other macrophage subsets. Mer Tyrosine Kinase (MerTK) is a major macrophage apoptotic cell marker and a member of the TAM (Tyro3, Axl, Mer) subfamily of receptors specifically involved in removal of early ACs [61,62]. MerTK makes M2c cells and M2c-like cells highly capable of clearing ACs with enhanced IL-10 secretion by M-CSF driven macrophages following Gas6 ligation and therefore maintaining anti-inflammatory conditions. M2d subsets are induced by TLR antagonists, leading to the release of IL-10 and VEGF, which promote tumor progression and angiogenesis [63]. Characteristics of M1 and M2 macrophages and their subtypes are summarized in Table 1.
Table 1:
Biological and physiological characteristics of M1 and M2 macrophages
| Phenotypes | Stimuli | Cytokines/Chemokines | Functions | References |
|---|---|---|---|---|
| M1 | IFN-γ, TNFα, LPS | IL-12, IL-1β, TNF-α, IL-6, NO, CXCL10, CCR7 | Embryo-implantation, Pro-inflammatory Th1 response, | [27,65] |
| M2a | IL-4, IL-13 | Fibronectin, Arginase-1, IL-10, IL-6, CCL-17, CCL-18 | Anti-inflammatory, Tissue remodeling/repair, Th2 activation | [68] |
| M2b | Immune complexes, TLR ligands, IL-1β | IL-10, IL-6, IL-1β, IL-12low | Immunoregulatory and Th2 activation | [68] |
| M2c | TGF-β, IL-10, glucocortic oids | IL-10, TGF-β, CCL16, CCL18, CXCL13 | Phagocytosis of apoptotic cells, Immunotolerance, matrix remodeling and repair | [68] |
| M2d | TLR ligands, adenosine receptor ligands | IL-10, VEGF | Tumor progression, proangiogenic | [68] |
Decidual macrophages also display polarization throughout pregnancy. M1 macrophages predominate during the peri-implantation period but transition to a mixture of M1 and M2 cells during vascular remodeling and trophoblast invasion and persisting until early second trimester [64]. During this time, placenta develops, ensuring prevention of fetal rejection. Also, around this time, the regulatory/homeostatic, immunomodulatory, and anti-inflammatory M2-like phenotype predominates promoting immune tolerance to the fetus for a successful pregnancy [14,18]. Regulation of macrophage differentiation during early pregnancy involves programmed death-1(PD-1)/PD-ligand-1 (PDL-1) signaling which promotes polarization toward the M2 phenotype [65]. When cultured in 1st trimester gravid serum, M1 macrophages switch to M2 and lose expression of procalcitonin (a marker for evaluating bacterial infections), suggesting that macrophages acquire different functional phenotypes different local environments [66] (Figure 2).
Figure 2: Macrophage polarization and M1/M2 macrophages during pregnancy.
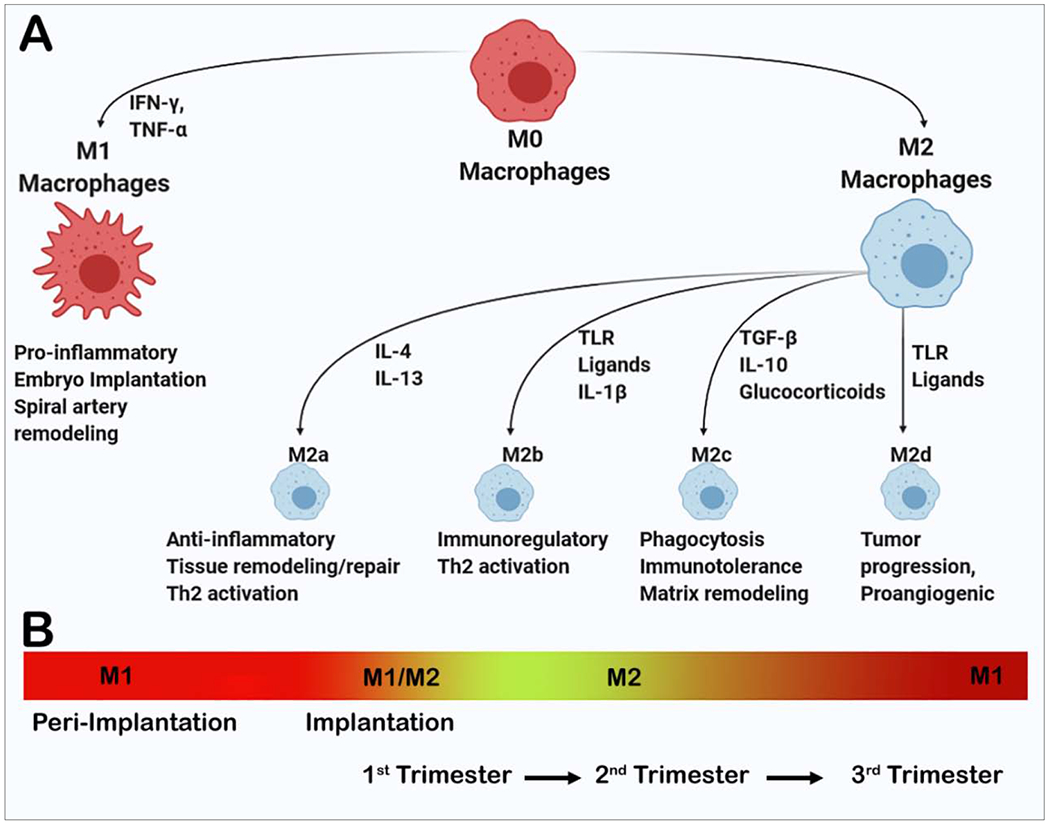
Macrophages polarize to M1 and M2a, M2b, M2c, and M2d phenotypes under different cytokine milieu (A). Macrophages display different phenotypes during pregnancy. M1 macrophages are dominant during peri-implantation stage. M1 and M2 both phenotypes are present during implantation and early 1st trimester. M2 phenotypes predominate during late first trimester and maintenance of pregnancy during 2nd trimester. M2 begin to decline and M1 again increase during 3rd trimester and parturition (B).
4.1. Macrophages in pregnancy complications
Alteration or perturbation of the M1/M2 balance and associated functions during pregnancy may result in complications, such as preeclampsia, fetal growth restriction, preterm delivery, and increased risk of future cardiovascular disease [67]. Preeclamptic women have fewer M2 macrophages, and most are classically activated M1 macrophages that secrete pro-inflammatory cytokines [68,69]. In preeclamptic pregnancies, macrophages express surface receptors characteristic of the M1 phenotype, suggesting that the systemic inflammation and excess cytokine production in these pregnancies may result from polarization of macrophages toward the M1 phenotype [68,70,71].
Furthermore, the myometrium in preeclamptic and preterm pregnancies show higher CD14+/CD68+ cells compared to decidua, where CD14-/CD68+ cells are more abundant [72]. In another study, the macrophages in the decidua basalis of preterm preeclamptic pregnancies, showed higher expression of CD14 and CD163 when compared with that of preterm control pregnancies [73].
Inappropriate macrophage polarization has also been associated with recurrent spontaneous abortion [74,75] and preterm labor [76]. In addition, macrophage dysregulation has been implicated in recurrent miscarriage and recurrent spontaneous abortion. Goto et al. in 2014 showed that decidual macrophages of recurrent miscarriage patients had decreased levels of Cathepsin E, which may be a trigger for miscarriage [77].
Another protein that may play a role in the regulation of decidual macrophage function, thromboplastin 1 (TSP1), is mainly expressed in platelets and can bind surface receptors such as CD36, CD47, and heparin sulfate proteoglycan on macrophages [78].
TSP-1 is a potent inducer of IL-10 expression in decidual macrophages, as shown in vitro, thereby implying it plays a role in IL-10 secretion and promotes immune tolerance at the placental-uterine interface [79]. In patients with recurrent spontaneous abortion, decreased TSP-1 expression and decreased IL-10 production in decidual macrophages has been observed, suggesting TSP-1 plays a crucial role in immune tolerance at the interface.
4.2. M1/M2 polarization and therapeutic potential
Macrophages are activated as pro-inflammatory M1 phenotypes in response to TLR ligands and IFN-γ. Contrary to this, they differentiate into homeostatic, regulatory, and anti-inflammatory M2 phenotype in response to IL-4 and IL-13 [55,59,60,80–82]. The ability to alter macrophage polarization suggests a promising therapeutic approach for inflammatory diseases specifically, by increasing M2 polarization. Several naturally occurring compounds have been shown to regulate M1 and M2 polarization and can be of therapeutic value for pregnancy-related and other disorders. For example, diosgenin glucoside (Dios), a saponin compound found in the extracts of Tritulus terrestris L strongly inhibits the expression of M1 markers and genes that promote inflammation in activated microglial cells [83]. Another compound, crocin, an active component of Crocus sativus L, exerts anti-inflammatory effects by inhibiting nuclear factor-kappa B (NF-κB) expression by suppressing NF-κB p65 translocation into the nucleus [84]. Crocin has been shown not only to suppress expression of inflammatory cytokines, such as TNF-α and IL-6, but also to promote expression of M2 markers, such as CD68 and CD206, and anti-inflammatory cytokines IL-10 and TGF-β. These results indicate that dios and crocin can be used as potential therapeutic agents in M1 and M2 polarization strategies. Several other compounds driving M1 to M2 polarization have demonstrated therapeutic potential. A few of them are capsaicin, lupeol or fargarsterol, malibatol A, and geraniin [85–88]. Using these strategies to modulate macrophage polarization at the maternal-fetal interface may alleviate pregnancy complications. In preeclampsia, non-classical monocyte-induced inflammation, as well as pro-inflammatory M1 macrophages predominate; with the use of M1 to M2 polarization modulators the effects of M1 dominance can be alleviated. These agents driving M1 to M2 polarization consequently may be highly effective anti-inflammatory therapeutic agents.
5. CONCLUSIONS
Macrophages play a significant role in immune tolerance to the fetal allograft via a variety of mechanisms. One aspect of immune tolerance derives from trophoblasts and endometrial cells, which secrete immunomodulatory hormones and regulate the expression of classical and non-classical MHC class I proteins. However, another major aspect of immune tolerance involves the remarkably plastic decidual macrophages. M1 macrophages promote embryo implantation during early pregnancy and are involved in presentation of foreign, pathogen-derived peptides to T cells for subsequent adaptive immune-mediated killing and thus protection of the fetus. M2 macrophages and their subsets induce an immunotolerant environment within the uterus for the fetus throughout pregnancy through a crosstalk with decidual NK cells and T cells. M1/M2 polarization is a unique feature of macrophages and the relative balance of these two phenotypes regulates the physiology of normal and pathologic pregnancies. They are heterogeneic but plastic and versatile cells which integrate multiple signals, including those from dead and damaged cells, debris, and the normal tissue microenvironment. Plasticity and switching of macrophage polarity could be therapeutically harnessed to develop anti-inflammatory approaches to treat pregnancy complications. More in-depth investigation of mechanisms by which M1/M2 phenotypes can be modulated by various compounds and their application in pathologic pregnancies will aid in development of novel therapeutics.
ACKNOWLEDGMENTS
The present work was supported by the NIH grant #R01HD088549 (the content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health).
List of abbreviations
- NK cells
natural killer cells
- TGF
Transforming growth factor
- IL
Interleukin
- T-reg
Regulatory T cells
- TNF
Tumor necrosis factor
- MHC
Major Histocompatibility Complex
- HLA
Human leukocyte antigen
- LILR
Leukocyte immunoglobulin-like receptor
- DC
Dendritic cells
- CSF
Colony stimulating factor
- GM-CSF
Granulocyte macrophage-colony stimulating factor
- VEGF
vascular endothelial growth factor
- IDO
Indoleamine 2,3 dioxygenase
- IFN-γ
Interferon-γ
- iNOS
Inducible nitric oxide synthase
- TLR
Toll-like receptor
- ACs
Apoptotic cells
- merTK
Mer Tyrosine Kinase
- PD-1
programmed death-1
- PDL-1
PD-ligand-1
- TSP1
Thromboplastin 1
- MMP
Matrix metalloproteinases
- PRRs
Pattern recognition receptors
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
Damage-associated molecular patterns
- NF-κB
Nuclear factor-kappa B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
Conflict of interest
The authors declare that they do not have any competing interests.
REFERENCES
- 1.Chen HL, Yang Y, Hu XL. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol 1991; 139:327–335. [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J 2005; 19:681–693. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S Regulatory T cells: Key controllers of immunologic self-tolerance. Cell 2000; 101: 455–458. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JS, Langat DL. HLA-G: a human pregnancy-related immunomodulators. Curr Opin Pharmacol 2009; 9(4):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilloy F, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. The Journal of experimental medicine. 1999;189:1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella M, Döhring C, Samaridis J, et al. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med. 1997;185(10):1743–1751. doi: 10.1084/jem.185.10.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DP, Jones DC, Anderson KJ, et al. The inhibitory receptor LILRB4 (ILT3) modulates antigen presenting cell phenotype and, along with LILRB2 (ILT4), is upregulated in response to Salmonella infection. BMC Immunol. 2009;10:56. Published 2009 Oct 27. doi: 10.1186/1471-2172-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houser BL. Decidual macrophages and their roles at the maternal-fetal interface. Yale J Biol Med 2012;85(1):105–118. [PMC free article] [PubMed] [Google Scholar]
- 9.Epelman S, Lavine KJ, Randolph GJ. 2014. Origin and functions of tissue macrophages. Immunity 41(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn TA, Chawla A, Pollard JW. 2013. Macrophage biology in development, homeostasis and disease. Nature 496(7446):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol (2006) 18(1):49–53. 10.1016/j.coi.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 12.Hunt JS. Stranger in a strange land. Immunol Rev 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel P The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution. Placenta. 2005. Sep-Oct;26(8-9):591–6. doi: 10.1016/j.placenta.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH. The role of decidual immune cells on human pregnancy. J Reprod Immunol. 2017. November;124:44–53. doi: 10.1016/j.jri.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019;4(31):eaat6114. doi: 10.1126/sciimmunol.aat6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010. June;63(6):434–44. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunt JS, Petroff MG, Burnett TG. Uterine leukocytes: key players in pregnancy. Semin Cell Dev Biol 2000. April;11(2):127–37. doi: 10.1006/scdb.2000.0158. [DOI] [PubMed] [Google Scholar]
- 18.Lidström C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol. 2003. December;50(6):444–52. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010. June;63(6):460–71. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 20.Renaud SJ, Graham CH. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol Invest. 2008;37(5):535–64. doi: 10.1080/08820130802191375. [DOI] [PubMed] [Google Scholar]
- 21.Li ZH, Wang LL, Liu H, Muyayalo KP, Huang XB, Mor G, Liao AH. Galectin-9 alleviates LPS-induced preeclampsia-like impairment in rats via switching decidual macrophage polarization to M2 subtype. Front Immunol. 2019. January 10;9:3142. doi: 10.3389/fimmu.2018.03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, Lash GE, Jenmalm MC, Ernerudh J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015. February 15;194(4):1534–44. doi: 10.4049/jimmunol.1401536. [DOI] [PubMed] [Google Scholar]
- 23.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Müller KH, Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996. July 15;98(2):482–9. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016. March;75(3):341–50. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 25.Krüssel J, Behr B, Hirchenhain J, Wen Y, Milki AA, Cupisti S, Bielfeld P, Polan ML. Expression of vascular endothelial growth factor mRNA in human preimplantation embryos derived from tripronuclear zygotes. Fertil Steril. 2000. December;74(6):1220–6. doi: 10.1016/s0015-0282(00)01581-8. [DOI] [PubMed] [Google Scholar]
- 26.Douglas NC, Zimmermann RC, Tan QK, Sullivan-Pyke CS, Sauer MV, Kitajewski JK, Shawber CJ. VEGFR-1 blockade disrupts peri-implantation decidual angiogenesis and macrophage recruitment. Vasc Cell. 2014. August 1;6:16. doi: 10.1186/2045-824X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler KC, Jena MK, Pradhan BS, et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS One. 2018;13(1):e0191040. Published 2018 Jan 11. doi: 10.1371/journal.pone.0191040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jena MK, Nayak N, Chen K, Nayak NR. Role of macrophages in pregnancy and related complications. Arch Immunol Ther Exp (Warsz). 2019. October;67(5):295–309. doi: 10.1007/s00005-019-00552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan X, Rai A, Kambham N, et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest. 2014;124(11):4941–4952. doi: 10.1172/JCI76864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jena MK, Sharma NR, Petitt M, Maulik D, Nayak NR. Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta. Biomolecules. 2020;10(6):953. Published 2020 Jun 24. doi: 10.3390/biom10060953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heikkinen J, Möttönen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003; 131(3):498–505. doi: 10.1046/j.1365-2249.2003.02092.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011. October 1;187(7):3671–82. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 33.Kämmerer U, Eggert AO, Kapp M, et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162(3):887–896. doi: 10.1016/S0002-9440(10)63884-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laskarin G, Cupurdija K, Tokmadzic VS, Dorcic D, Dupor J, Juretic K, Strbo N, Crncic TB, Marchezi F, Allavena P, Mantovani A, Randic Lj Rukavina D. The presence of functional mannose receptor on macrophages at the maternal-fetal interface. Hum Reprod. 2005. April;20(4):1057–66. doi: 10.1093/humrep/deh740. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson C, Mjösberg J, Matussek A, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One. 2008;3(4):e2078. Published 2008 Apr 30. doi: 10.1371/journal.pone.0002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006. September;12(9):1065–74. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 37.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54(2-3):281–94. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 38.Harris LK, Keogh RJ, Wareing M, et al. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169(5):1863–1874. doi: 10.2353/ajpath.2006.060265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003. January;24(1):53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 40.Imanishi T, Hano T, Nishio I, Han DK, Schwartz SM, Karsan A. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from endothelial cells. Jpn Circ J. 2001. June;65(6):556–60. [DOI] [PubMed] [Google Scholar]
- 41.Imanishi T, Han DK, Hofstra L, Hano T, Nishio I, Liles WC, Gown AM, Schwartz SM. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from monocytes/macrophage. Atherosclerosis. 2002. March;161(1):143–51. [DOI] [PubMed] [Google Scholar]
- 42.Engert S, Rieger L, Kapp M, Becker JC, Dietl J, Kämmerer U. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am J Reprod Immunol. 2007. August;58(2):129–37. doi: 10.1111/j.1600-0897.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 43.Hazan AD, Smith SD, Jones RL, Whittle W, Lye SJ, Dunk CE. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol. 2010. August;177(2):1017–30. doi: 10.2353/ajpath.2010.091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986. October;93(10):1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 45.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004. April;51(4):275–82. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 46.Tang MX, Hu XH, Liu ZZ, Kwak-Kim J, Liao AH. What are the roles of macrophages and monocytes in human pregnancy? J Reprod Immunol. 2015. November;112:73–80. doi: 10.1016/j.jri.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 48.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014. November 20;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Xie X, He H, Colonna M, Seya T, Takai T, Cray BA. Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod. 2005. September;73(3):510–8. doi: 10.1095/biolreprod.104.033951. [DOI] [PubMed] [Google Scholar]
- 50.Pollard JW. Role of colony-stimulating factor-1 in reproduction and development. Mol Reprod Dev. 1997. January;46(1):54–60; discussion 60-1. doi: . [DOI] [PubMed] [Google Scholar]
- 51.Manaster I, Mizrahi S, Goldman-Wohl D, et al. Endometrial NK cells are special immature cells that await pregnancy. Journal of Immunology. 2008; 181 (3): 1869–1876 [DOI] [PubMed] [Google Scholar]
- 52.Grozdics E, Berta L, Bajnok A, et al. B7 costimulation and intracellular indoleamine-2,3-dioxygenase (IDO) expression in peripheral blood of healthy pregnant and non-pregnant women. BMC Pregnancy Childbirth. 2014; 14:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sayama S, Nagamatsu T, Schust DJ, et al. Human decidual macrophages suppress IFN-γ production by T cells through costimulatory B7-H1:PD-1 signaling in early pregnancy. Journal of Reproductive Immunology. 2013; 100(2): 109–117. [DOI] [PubMed] [Google Scholar]
- 54.Miwa N, Hayakawa S, Miyazaki S, Myojo S, Sasaki Y, Sakai M, et al. . IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-γ increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod. (2006) 11:865–70 [DOI] [PubMed] [Google Scholar]
- 55.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology 25(12):677–686. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. 2013. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of pathology 229(2):176–185. [DOI] [PubMed] [Google Scholar]
- 57.Sica A, Mantovani A. 2012. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation 122(3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon S Alternative activation of macrophages. Nat Rev Immunol. 2003. January;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 59.Gough MJ, Melcher AA, Ahmed A, Crittenden MR, Riddle DS, Linardakis E, Ruchatz AN, Emiliusen LM, Vile RG. Macrophages orchestrate the immune response to tumor cell death. Cancer Res. 2001. October 1;61 (19):7240–7. [PubMed] [Google Scholar]
- 60.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008. January 1;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 61.Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 2019;10:792. Published 2019 Apr 15. doi: 10.3389/fimmu.2019.00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189(7):3508–3520. doi: 10.4049/jimmunol.1200662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation. 2013. August;36(4):921–31. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaiswal MK, Mailers TM, Larsen B, Kwak-Kim J, Chaouat G, Gilman-Sachs A, Beaman KD. V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction. 2012. May; 143(5):713–25. doi: 10.1530/REP-12-0036. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Ma L, Hu X, Ji J, Mor G, Liao A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum Reprod. 2019. January 1;34(1):25–36. doi: 10.1093/humrep/dey347. [DOI] [PubMed] [Google Scholar]
- 66.Rami D, La Bianca M, Agostinis C, Zauli G, Radillo O, Bulla R. The first trimester gravid serum regulates procalcitonin expression in human macrophages skewing their phenotype in vitro. Mediators Inflamm. 2014;2014:248963. doi: 10.1155/2014/248963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duley L The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009. June;33(3): 130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 68.Ning F, Liu H, Lash GE. The role of decidual macrophages during normal and pathological pregnancy. Am J Reprod Immunol. 2016. March;75(3):298–309. doi: 10.1111/aji.12477. [DOI] [PubMed] [Google Scholar]
- 69.Faas MM, Spaans F, De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front Immunol. 2014. June 30;5:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown MB, von Chamier M, Allam AB, Reyes L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol. 2014. November 24;5:606. doi: 10.3389/fimmu.2014.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams Z Inducing tolerance to pregnancy. N Engl J Med. 2012;367(12): 1159–1161. doi: 10.1056/NEJMcibr1207279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JS, Romero R, Cushenberry E, Kim YM, Erez O, Nien JK, Yoon BH, Espinoza J, Kim CJ. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta. 2007. May-Jun;28(5-6):571–6. doi: 10.1016/j.placenta.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Schonkeren D, van der Hoorn ML, Khedoe P, et al. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol. 2011;178(2):709–717. doi: 10.1016/j.ajpath.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsao FY, Wu MY, Chang YL, Wu CT, Ho HN. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J Formos Med Assoc. (2017) 117:204–11. [DOI] [PubMed] [Google Scholar]
- 75.Kolben TM, Rogatsch E, Vattai A, et al. PPARγ Expression is diminished in macrophages of recurrent miscarriage placentas. Int J Mol Sci. 2018;19(7):1872. Published 2018 Jun 26. doi: 10.3390/ijms19071872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y, Romero R, Miller D, et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by Rosiglitazone treatment. J Immunol. 2016;196(6):2476–2491. doi: 10.4049/jimmunol.1502055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goto S, Ozaki Y, Suzumori N, Yasukochi A, Kawakubo T, Furuno T, Nakanishi M, Yamamoto K, Sugiura-Ogasawara M. Role of cathepsin E in decidual macrophage of patients with recurrent miscarriage. Mol Hum Reprod. 2014. May;20(5):454–62. doi: 10.1093/molehr/gau008. [DOI] [PubMed] [Google Scholar]
- 78.Yamauchi Y, Kuroki M, Imakiire T, Uno K, Abe H, Beppu R, Yamashita Y, Kuroki M, Shirakusa T. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 2002. August;21(5):441–8. doi: 10.1016/s0945-053x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 79.Jin Y, Wang X, Xiao Y, Lv C, Ding C, Lin Q. The role of TSP-1 on decidual macrophages involved in the susceptibility to unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2009. March;61(3):253–60. doi: 10.1111/j.1600-0897.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 80.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003. February;73(2):209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 81.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004. March 30;101(13):4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000. June 15;164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 83.Wang S, Wang F, Yang H, Li R, Guo H, Hu L. Diosgenin glucoside provides neuroprotection by regulating microglial M1 polarization. Int Immunopharmacol. 2017. September;50:22–29. doi: 10.1016/j.intimp.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Li J, Lei HT, Cao L, Mi YN, Li S, Cao YX. Crocin alleviates coronary atherosclerosis via inhibiting lipid synthesis and inducing M2 macrophage polarization. Int Immunopharmacol. 2018. February;55:120–127. [DOI] [PubMed] [Google Scholar]
- 85.Bok E, Chung YC, Kim KS, Baik HH, Shin WH, Jin BK. Modulation of M1/M2 polarization by capsaicin contributes to the survival of dopaminergic neurons in the lipopolysaccharide-lesioned substantia nigra in vivo. Exp Mol Med. 2018. July 3;50(7):1–14. doi: 10.1038/s12276-018-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Y, Li X, Chen J, Chen T, Shi Z, Lei M, Zhang Y, Bai P, Li Y, Fei X. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int Immunopharmacol. 2016. January;30:74–84. doi: 10.1016/j.intimp.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 87.Pan J, Jin JL, Ge HM, Yin KL, Chen X, Han LJ, Chen Y, Qian L, Li XX, Xu Y. Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARγ-dependent manner. J Neuroinflammation. 2015. March 14; 12:51. doi: 10.1186/s12974-015-0270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X, Li J, Peng X, Lv B, Wang P, Zhao X, Yu B. Geraniin inhibits LPS-induced THP-1 macrophages switching to M1 phenotype via SOCS1/NF-κB pathway. Inflammation. 2016. August;39(4): 1421–33. doi: 10.1007/s10753-016-0374-7. Erratum in: Inflammation. 2020 Oct 7 [DOI] [PubMed] [Google Scholar]


