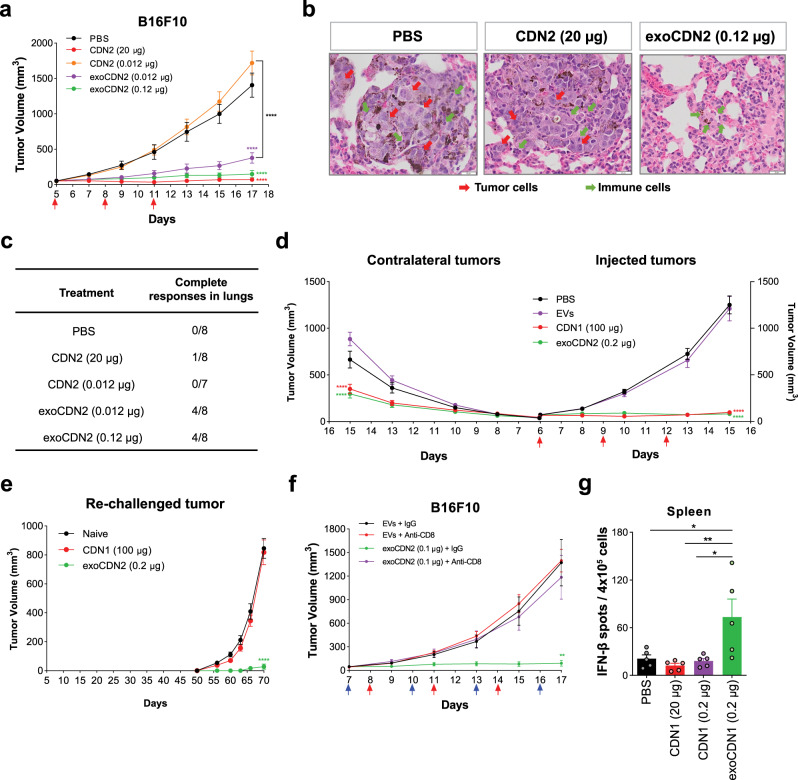
Fig. 2. exoSTING elicits strong anti-tumor responses with systemic tumor-specific immune activation in a B16F10 tumor model.
a–c B16F10 tumor cells (1 × 106 cells) were implanted subcutaneously in right flank of C57BL/6 mice. At Day 4, B16F10 tumor cells (1 × 105 cells) were injected intravenously to induce lung metastasis. PBS, free CDN2 (20 or 0.012 µg) and exoCDN2 (0.012 or 0.12 µg) were injected into subcutaneous tumors (n = 8 animals per group). Tumor growth curves of subcutaneous tumors (a), representative H&E stained images from PBS, CDN2 (20 µg), and exoCDN2 (0.12 µg) (b), and number of complete responders by histopathological analysis (c). Complete remission defined by pathologist from H&E staining of whole lungs. d B16F10 tumor cells were implanted subcutaneously in right (1 × 106 cells) and left (5 × 105 cells) flanks of mice (n = 10 animals per group). PBS, unloaded EVs, CDN1 (100 µg), exoCDN2 (0.2 µg) were injected intratumorally into right flank tumors and both injected and non-injected contralateral tumor growth was measured. e Subcutaneous B16F10 tumors were treated with PBS (n = 5 animals), unloaded EVs (n = 5 animals), CDN1 (100 µg) (n = 10 animals), and exoCDN2 (0.2 µg) (n = 15 animals). B16F10 cells (1 × 106 cells) were implanted to mice that had CR and naïve mice (n = 5 animals) on day 50 to the left flank. Re-challenged tumor growth was measured. f Tumor growth inhibition after CD8 T cell depletion. B16F10 tumor bearing mice received the IgG (10 mg/kg) or anti-CD8 antibody (10 mg/kg) intraperitoneally, one day before IT injection (n = 5 animals per group). Blue arrows indicate intraperitoneal injection days. g Tumor specific IFN-γ response after PBS, CDN1 (0.2 or 20 µg), and exoCDN1 (0.2 µg) treatment to B16F10 tumor (n = 5 animals per group). Data are presented as means ± s.e.m from replicate samples as indicated. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 by one-way ANOVA for g and two-way ANOVA with Tukey’s multiple comparison test for tumor growth studies.