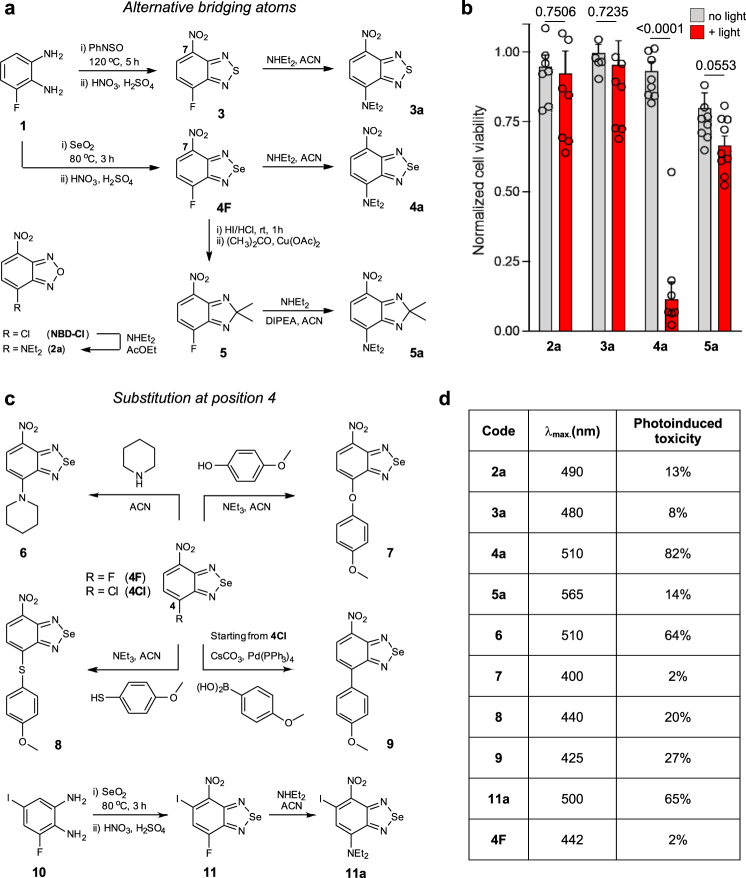
Fig. 1. Synthetic routes for the preparation of small photosensitizers.
a Condensation of 1 with PhNSO or SeO2 followed by nitration led to compounds 3 and 4F, respectively. Reduction of 4F followed by Cu-catalyzed addition of acetone rendered compound 5. Nucleophilic substitutions of 2–5 with diethylamine yielded 2a–5a. b Human U87 cells were incubated with 2a–5a (100 μM) in Krebs–Ringer buffer and illuminated (red bars) or not (gray bars) with a ThorLabs M530L3 LED (10 mW, 37 J cm−2, red). Cell viability was assessed 16 h post illumination using a TACSR MTT cell proliferation assay with values normalized to the viability of untreated cells. Data presented as mean values ± SEM (n = 3 independent experiments). c Nucleophilic substitution of 4F with piperidine, 4-methoxyphenol, and 4-mehtoxythiophenol led to compounds 6, 7, and 8, respectively. Suzuki coupling of 4Cl with methoxybenzene-4-boronic acid rendered compound 9. Condensation of compound 10 with SeO2 followed by nitration and nucleophilic substitution resulted in compound 11a. d Maximum excitation wavelengths and phototoxicity of compounds 2a–11a and 4F in human cells as determined in (b). Values indicate the decrease in viability between irradiated and non-irradiated cells after incubation with compounds 2a–11a (100 μM). P values were obtained from two-tailed unpaired t tests. Source data are available.