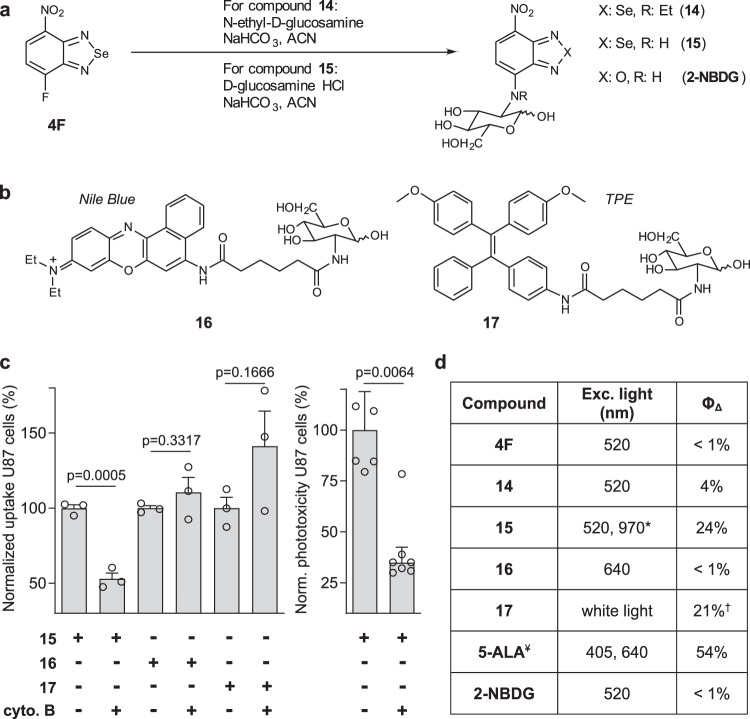
Fig. 3. d-Glucose derivatives of benzoselenadiazole — but not of other photosensitizers—are recognized by GLUT transporters.
a Chemical synthesis of the d-glucose aminobenzodiazole compounds 14 and 15, and the non-photosensitive control compound 2-NBDG. b Structures of d-glucose derivatives of the photosensitizers Nile Blue (compound 16) and tetraphenylethylene (TPE, compound 17). Full synthetic and characterization details described in the Supplementary Information. c Flow cytometric quantification (gating: Supplementary Fig. 5) of uptake of d-glucose derivatives in human U87 cells (200,000 cells/well) after incubation for 1 h with compounds 15–17 (50–100 μM) in Krebs–Ringer buffer in the presence or not of the GLUT inhibitor cytochalasin B (20 μM) (λexc/em 488/610 nm (15), 635/670 nm (16), 355/450 nm (17)). Phototoxicity in U87 cells after incubation with compound 15 (100 μM) in the presence or absence of cytochalasin B (20 μM). Cells were illuminated (10 mW, 37 J cm−2) and viability was assessed 16 h post illumination using a TACSR MTT cell proliferation assay with values normalized to those in cells without cytochalasin B. Data presented as mean values ± SEM (n = 3 independent experiments). d Excitation wavelengths used for single-photon illumination (*corresponds to two-photon illumination) and singlet oxygen generation quantum yields determined using DPBF in EtOH (Note: DPBF was found insoluble in water) using Rose Bengal as a reference (68%)61. †Singlet oxygen generation quantum yield reported for TPE62. ¥Reported values for protoporphyrin IX, the main photosensitizer produced upon metabolism of 5-aminolevulinic acid (5-ALA)63. P values were obtained from two-tailed unpaired t tests. Source data are available.