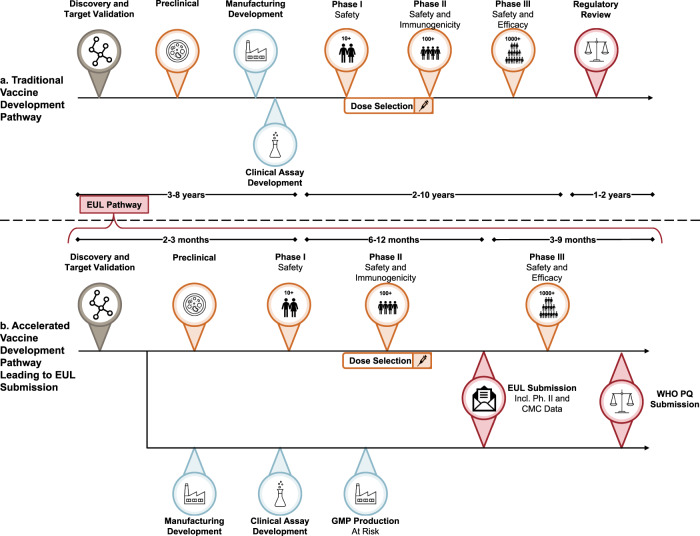
Fig. 1. Potential for acceleration across all aspects of development for an EUL submission.
Comparison of the traditional drug and vaccine development timeline adapted from Heaton4 (a) with an idealized vaccine development timeline targeting World Health Organization Emergency Use Listing submission based on nOPV2 and COVID-19 experiences (b). Symbols are used to indicate key stages in the vaccine development process; orange-red shading for clinical and blue shading for CMC activities.