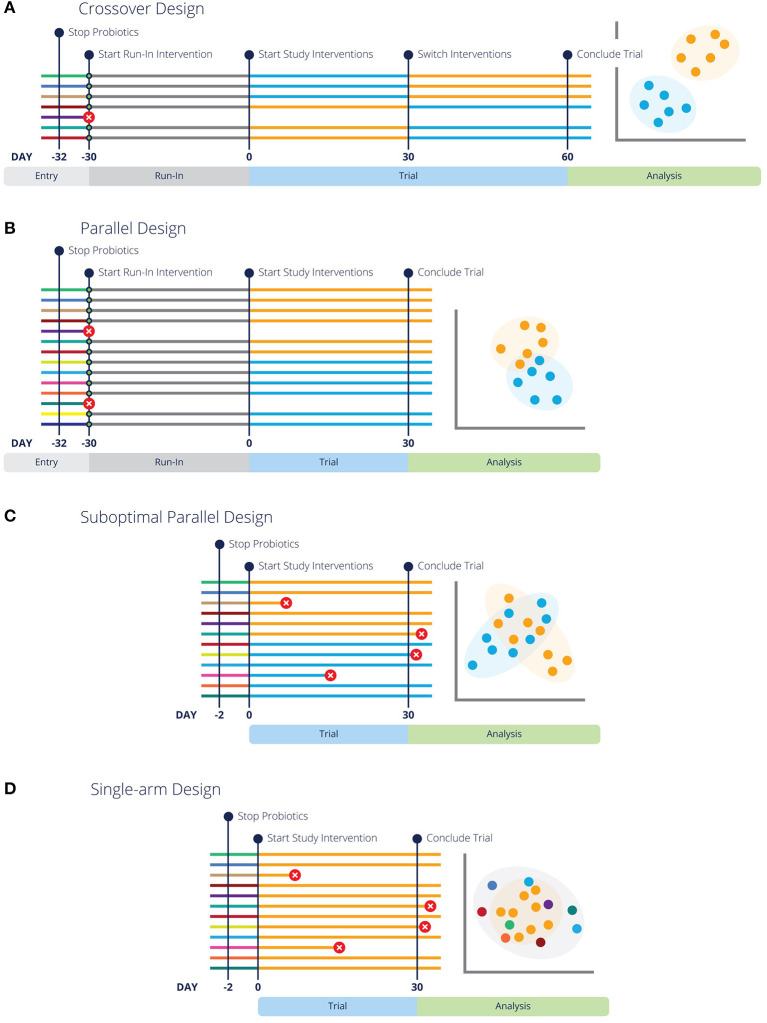
Figure 1.
Different experimental design strategies for feeding trials or clinical trials are more likely to have conclusive results with run-in periods and pre-screening. Each line represents a different subject in the designated trial, with colors representing respective baseline, run-in and study interventions. Green circles indicate pre-screening tests. Lines with a red circle indicate the subject is rejected by screening criteria or did not complete the trial. The analysis graphic resembles a PCoA plot (a common representation of microbiome data), with each data point representing an individual in the study. Data points are colored by the diet or intervention at the time of sampling. (A,B) Illustrate optimal crossover and parallel study design, highlighting that entry screening and run-in periods lead to fewer dropouts and more conclusive results. Additionally, (B) Highlights the need for more subjects in a parallel design study. (C) Represents the same parallel design as (A), but without pre-screening or a washout period, which lends to inconclusive results. Similarly, (D) illustrates a “Before vs. After” design without pre-screening or a washout period and inconclusive results.