Fig. 3.
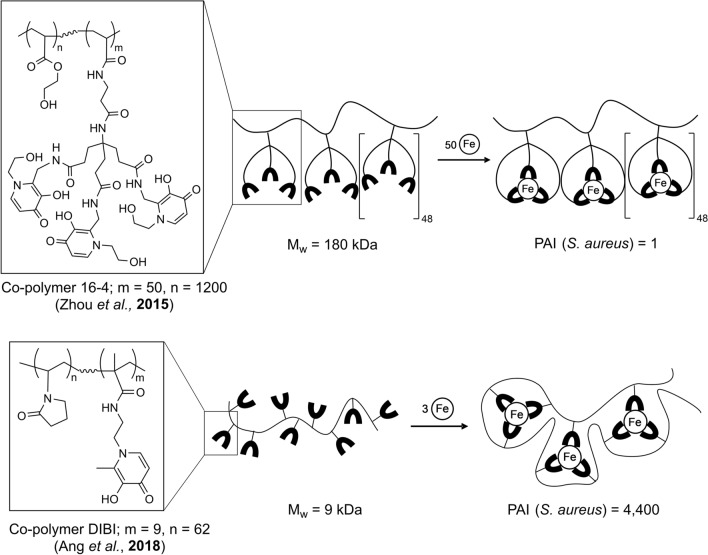
Structural differences between co-polymer 16–4 (Zhou, Kong, et al. 2015a, b) and co-polymer DIBI (Ang et al. 2018). For co-polymer 16–4, each of the metal binding monomer units used to prepare the co-polymer were capable of hexadentate iron binding. For DIBI, monomer groups capable of only bidentate iron binding were utilized. However, the metal binding characteristics and antimicrobial activities of the resultant co-polymers were strikingly different. The tertiary structure of DIBI appears such that it folds and wraps itself around its bound iron as three neighboring metal binding groups cooperate to complete full hexadentate iron coordination as shown. In contrast, with co-polymer 16–4 cooperation of neighboring monomer groups is not required and the co-polymer likely remains as a linear molecule with iron bound by individual monomer groups as shown. These two different structures result in very different overall iron binding strengths and corresponding antimicrobial activities as reflected in their MIC values for S. aureus. Comparisons of component monomer and resultant copolymer MIC values allows the quantification of the gain in antimicrobial activity achieved with the co-polymer structure over its component metal binding monomer as shown by a measured Polymer Activity Index (PAI). PAI = 1 for 16–4 polymer which indicates no net improvement or degradation of the antimicrobial activity over the free monomer. PAI = 4400 for DIBI, indicating a very strong enhancement of antimicrobial activity of the polymer over the free monomer (figure adapted from Gumbau-Brisa et al. 2020)