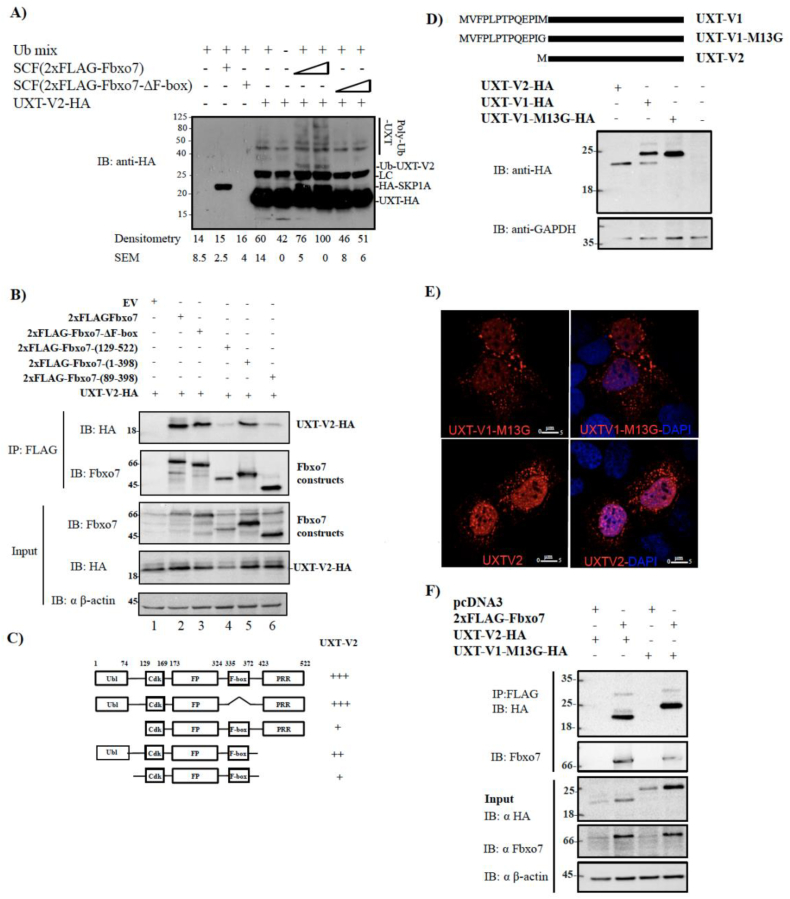
Fig. 1.
Fbxo7 ubiquitinates UXT-V2 in vitro and interacts with UXT-V2 and UXT-V1 in vivo. A) In vitro ubiquitination assays using purified SCF(2xFLAG-Fbxo7) or 2xFLAG-Fbxo7 lacking the F-box domain in two different concentrations (25 and 50 nM) and purified UXT-V2 from HEK293T cells as a substrate. The samples were used for western blotting, and an anti-HA antibody was used to visualize polyubiquitinated UXT—V2. The smear of each band was quantified by densitometry using Image J and the +/− SEM of the triplicates was calculated by GraphPad Prism; LC (light chain). B) Extracts of HEK293T cells transfected with the indicated 2xFLAG-Fbxo7 plasmids and UXT-V2-HA were immunoprecipitated with agarose anti-FLAG. Input and eluted proteins were subjected to immunoblotting with the indicated antibodies. C) Summary of the interaction mapping between UXT-V2 and Fbxo7 proteins. D) Representation of UXT—V1, UXT-V1-M13G and UXT-V2 isoforms and their expression in HEK293T cells transfected with each plasmid. E) Multiphoton microscopy of U2OS transfected with UXT-V1-M13G-HA or UXT-V2-HA. The slides were incubated with anti-HA and nuclei were probed by DAPI. F) HEK293T cells were transfected with FLAG-Fbxo7 and UXT-V1-M13G or UXT—V2. The cellular extracts were immunoprecipitated, resolved by SDS-PAGE and probed with the indicated antibodies. All the inputs represent 3% of the total protein used in coimmunoprecipitation assays. All cell lysates were obtained by NP-40 lysis buffer.