Abstract
NK cells change their phenotype and functional characteristics during activation. In this work, we searched for a relationship of HLA-DR expression with differentiation stages and functional activity of NK cells ex vivo and stimulated in vitro with IL-2 challenged with gene modified feeder K562 cells expressing membrane-bound IL-21 (K562-mbIL21). This stimulation technique has been described for NK cell expansion in clinical use. We have observed that HLA-DR expression in freshly isolated circulating NK cells was mostly associated with less differentiated CD56brightCD57− cells, although in some individuals it could also be found in terminally differentiated CD57+ cells. Ex vivo HLA-DR+ NK cells possessed better capacity to produce IFN-γ in response to cytokine stimulation compared to their HLA-DR− counterparts. In vitro activation with IL-2 and K562-mbIL21 induces an increase in HLA-DR-positive NK cell proportion, again mostly among CD56brightCD57− NK cells. This happened in particular due to appearance of HLA-DR+ expression de novo in HLA-DR-negative cells. Acquired in vitro HLA-DR expression was associated with NK cell proliferation activity, more intense cytokine-induced IFN-γ production, increased degranulation toward feeder cells, and higher expression of CD86 and NKG2D. Thus, stimulation with IL-2/K562-mbIL21 causes a significant phenotype and functional shift during NK cell activation and expansion.
Keywords: Human NK cells, HLA-DR, IL-2, IL-21, NK cell activation
INTRODUCTION
NK cell differentiation is regulated by a large set of cytokines produced by surrounding cells, as well as by direct cell-to-cell contacts. Cytokines and chemokines direct the migration of NK cells, their proliferation, and functional activity.1,2 As short-lived white blood cells, NK cells do not proliferate in the absence of activating cytokines in the medium and die shortly afterward. NK cell activation and differentiation caused by external stimuli is accompanied by change in expression of certain internal and surface proteins.3,4 It was observed that NK cell activation often leads to appearance of HLA-DR on the cell surface.5–7 This molecule is associated with antigen-presenting cells, as it is directly involved in the process of antigen presentation to CD4+ T cells.8 In healthy people a small fraction of circulating NK cells expresses HLA-DR.9 This circulating fraction of HLA-DR-positive NK cells may substantially increase under conditions associated with chronic inflammation, such as HIV-caused immunodeficiency,10,11 multiple sclerosis,12 IgA-nephropathy.5 HLA-DR expression in NK cells also increases under bacillus Calmette–Guérin stimulation.13 This indicates the physiological importance of the HLA-DR-positive cells in vivo. However, this subset is still poorly characterized. In particular, it remains unclear whether the HLA-DR expression is connected to NK cell differentiation. The functional significance of HLA-DR itself on NK cells is still under discussion. By now, it has been shown that NK cells can present certain antigens to T cells with the use of this molecule, for example tetanus toxin,14 and can provide costimulatory signal for central memory T cell differentiation.15 Still, HLA-DR may be a ligand for an unknown receptor itself. By now, HLA-DR expression on activated NK cells can be associated with enhanced proliferation ability13 and with elevated cytotoxic activity against certain target cells.9,13 It is not fully understood what kind of stimuli can induce HLA-DR expression, as well as whether HLA-DR marks the activated state of NK cells in general, or only under certain conditions, and how NK cell characteristics change together with HLA-DR expression. It is also unclear, whether the growth of HLA-DR-positive NK cell fraction during activation happens due to proliferation of HLA-DR+ cells observed in blood, or due to an induction of HLA-DR expression de novo in mature NK cells. There is now evidence in favor of the first hypothesis, provided by Evans et al., 2011, but the second scenario should not be excluded.
It is known that in vitro IL-2 can increase the proportion of NK cells expressing HLA-DR.13,14 Also, it has been recently shown that combination of IL-2 and IL-21 can increase this proportion to even greater extent.15 IL-2 and other cytokines, such as IL-15, IL-21, are important tools in studying NK cells, as they allow maintaining the viability and growth of the cells in culture up to several weeks.16–18 This response to cytokines is widely used to expand NK cells ex vivo for their further applications in adoptive immunotherapy.19–21 For this purpose, soluble cytokines can be used, but much more impressive results have been acquired using feeder cells (K562, EBV cell lines), modified to express membrane-bound cytokines and other activating molecules, such as 4–1BBL and MICA,20 mbIL-15 and 4–1BBL.22,23 Addition of feeder cells to the cultures led to a significant increase in NK cell expansion. Genetic modification of feeder cells causing them to express stimulatory cytokines, such as IL-15 or IL-21, promoted survival of NK cells.22,24 This technique is currently used for obtaining high numbers of NK cells for adoptive immunotherapy in clinic.23,25
In one of the recent works the researchers describe a method which allowed them to gain 108-fold expansion of NK cell progeny in 42 days.24 In this method, IL-2 was used in combination with membrane-bound IL-21, exposed on the surface of K562 cell clone (K562-mbIL21). K562 cells are often used as targets for natural killers, as they effectively induce their functional activity and cytotoxicity. In this work, we found out that the use of K562-mbIL21 cells supplemented with IL-2 for NK cell stimulation led to extremely high expression of HLA-DR in NK cells. This approach allowed to demonstrate that HLA-DR expression can be induced de novo in mature NK cells isolated from peripheral blood. We found that HLA-DR+ NK cells obtained in this stimulation model consist mainly of strongly proliferating CD56brightCD57−NKG2A+ cells, in compliance with the results of ex vivo NK cell analysis: we have shown that in freshly isolated blood NK cells HLA-DR expression can also be found mostly on less differentiated CD56brightCD57− cells. However, in some individuals CD56dimCD57+HLA-DR+ cells were observed, which comprise terminally differentiated but at the same time activated NK cells. HLA-DR-positive ex vivo NK cells demonstrated increased IFN-γ production in response to cytokine stimulation. HLA-DR-positive NK cells obtained during IL-2/K562-mbIL21 stimulation also displayed high level of IFN-γ production, as well as high degranulation and upregulation of several activation markers, including CD86, thus acquiring signaling mechanisms for both enhanced killing and possible antigen presentation. Such change in the NK cells characteristics during multiday cultivation should be considered when developing methods of using stimulated NK cells in immunotherapy. Collectively, our data suggest that HLA-DR expression is not simply a marker of general NK cell activation or proliferation; it is induced upon stimulation of certain intracellular pathways alongside with other markers expression, IFN-γ production and degranulation, which altogether results into appearance of circulating NK cells with enhanced effector functions.
RESULTS
HLA-DR surface expression in peripheral blood NK cells at various stages of their differentiation
NK cells ranged from 7 to 25% of all mononuclear cells in all studied volunteers (30 individuals). The percentage of HLA-DR-positive NK cells among all NK cells circulating in the peripheral blood was approximately 6.0 ± 4.6%. It should be noted that the expression level of HLA-DR on the surface of nonactivated NK cells was rather low compared to monocytes or B cells, which influences the accuracy of HLA-DR+ cell proportion calculation. After magnetic separation the purity of NK cells fraction reached 95–99%. Isolated cells mainly had phenotype CD56+, with marginal counts of CD3+, CD14+, or CD20+cells (less than 0.5% for each type) (Figure 1a). Proportion of CD56neg cells in different samples was within the range of 0.2%–5%. Admixture of CD56+CD7− cells routinely constituted not more than 0.7% (Figure 1a).
Figure 1.
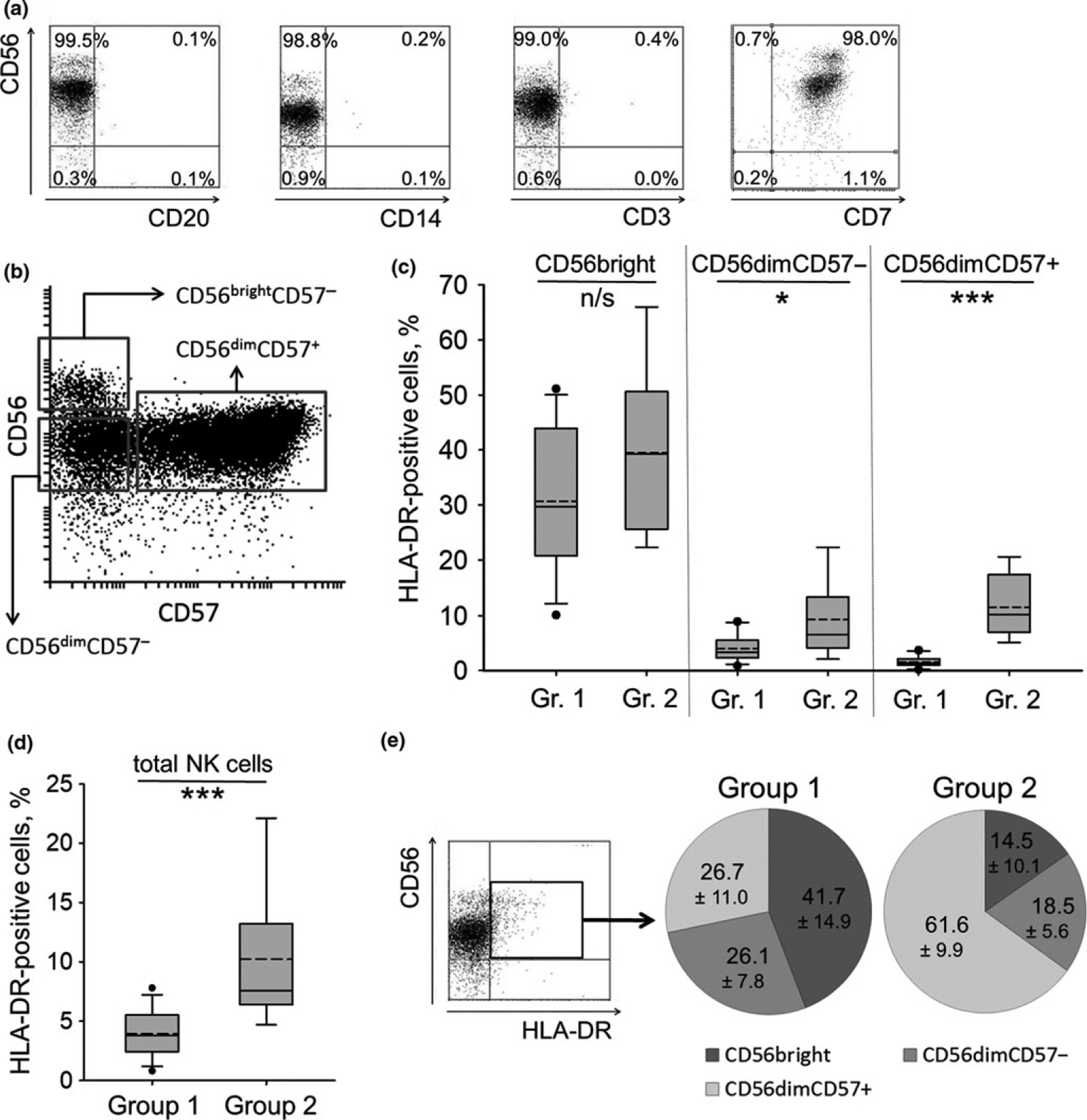
Analysis of HLA-DR expression in freshly isolated NK cells. (a) NK cell phenotype measured directly after magnetic separation. (b) NK cell subsets, gated to analyze HLA-DR expression, on different stages of differentiation. (a), (b) Representative staining obtained from a single donor out of 21 donors examined is shown. (c, d) Box plots illustrating proportion of HLA-DR-positive cells in CD56bright, CD56dimCD57− and CD56dimCD57+ NK cells (c) and in total NK cells (d) in donors from group 1 and 2. Group 1: proportion of HLA-DR+ cells in CD56dimCD57+ subset is less than 5%; group 2: proportion of HLA-DR+ cells in CD56dimCD57+ subset is more than 5%. Whiskers display the 5th and 95th percentiles, solid line — the median, dashed line — the mean value, black dots — the outliers. Gr. 1 and Gr. 2 stand for group 1 and 2, and comprise 12 and 9 donors, respectively. (e) Proportions of NK cells on different stages of differentiation within HLA-DR-positive subpopulation in donors from group 1 and 2. Mean value ± SD out of 12 and 9 donors examined, respectively, is shown.
Firstly, we analyzed a relationship between HLA-DR surface expression level in peripheral blood NK cells and stages of NK cell differentiation, which were determined according to CD56 and CD57 expression (Figure 1b). It is commonly accepted that CD56bright NK cells are less differentiated cells compared to CD56dim cells.26,27 CD57 is expressed on mature NK cells as the marker of the final stage of their differentiation.28 The HLA-DR surface expression was characterized in the following subpopulations: CD56bright, CD56dimCD57−, CD56dimCD57+ (Figure 1c–e, Supplementary table 1). The proportion of HLA-DR-positive cells in peripheral blood was much higher in CD56bright cells than in CD56dim cells for the most part of the volunteers (Figure 1c, group 1). However, in some individuals we observed relatively high proportion of HLA-DR-positive NK cells and HLA-DR expression intensity in CD56dimCD57+ cells (sometimes together with CD56dimCD57− subset) unlike with group 1 (Figure 1c, group 2). This phenomenon was associated with the increased levels of total HLA-DR-positive NK cells in the peripheral blood in most of the individuals from group 2 (Figure 1d). Inside the HLA-DR-positive subpopulation, due to almost two times increased proportion of HLA-DR+CD56dimCD57+ cells in these donors, the proportion of HLA-DR+CD56bright cells was reduced (Figure 1e, group 2). In other individuals, HLA-DR-positive NK cells mostly consisted of cells with CD56bright phenotype (Figure 1e, group 1).
To conclude, the highest level of HLA-DR expression in NK cell subsets was observed in less differentiated CD56bright cells. In terminally differentiated CD56dimCD57+ cells, part of the volunteers had very low level of HLA-DR-positive cells, but in certain individuals this level was significantly increased.
Ex vivo HLA-DR-positive NK cells produce more IFN-γ but are less cytotoxic in response to cytokine stimulation compared to HLA-DR− counterparts
There is little information in literature about functional activity of circulating HLA-DR-positive NK cells in vivo or ex vivo. In one of the studies, it was revealed that freshly isolated HLA-DR+ NK cells showed more efficient cytotoxicity toward NK-resistant targets than HLA-DR− cells.9 In this work, we have evaluated capacity of ex vivo HLA-DR-positive NK cells to produce IFN-γ in response to IL-12+IL-15 stimulation, and their antibody-dependent or natural cytotoxicity enhanced by IL-2. We observed that CD56dimHLA-DR+ NK cells, freshly isolated and enriched by cell sorting, demonstrated higher level of IFN-γ production than their HLA-DR− counterparts (Figure 2a and b; Supplementary figure 1, 2a). CD56bright HLA-DR+ and HLA-DR− NK cells both produced IFN-γ in response to cytokine stimulation very intensively, either in secreted IFN-γ concentrations or in cell percentage, which is typical for CD56bright subpopulation.
Figure 2.
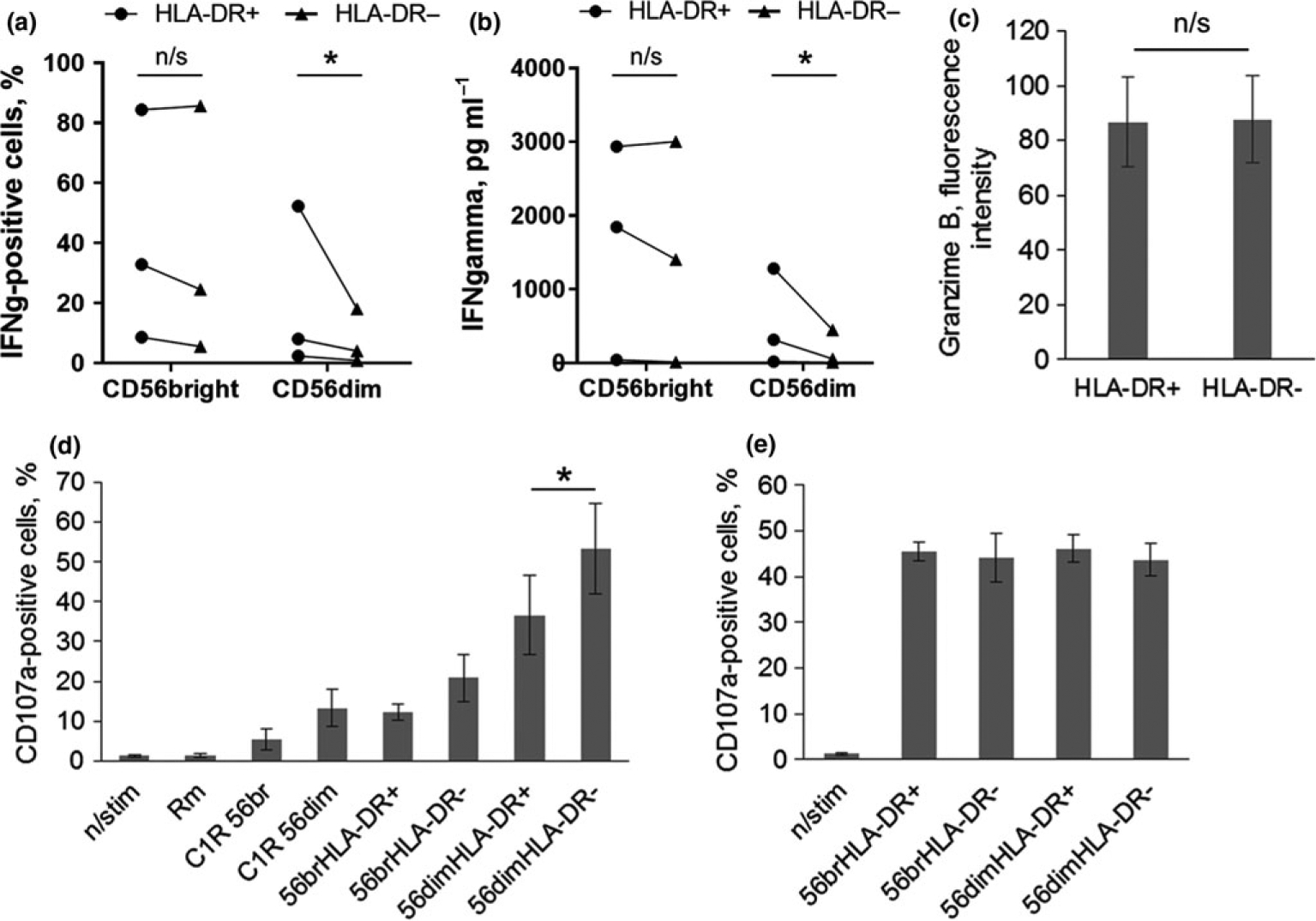
Analysis of IFN-γ production and cytotoxic activity of freshly isolated enriched CD56brightHLA-DR−, CD56brightHLA-DR+, CD56dimHLA-DR− and CD56dimHLA-DR+ NK cells. (a, b) IFN-γ production was measured by intracellular staining (a) and ELISA essay (b), prior to that cells were stimulated with IL-12 and IL-15 (10 ng mL−1, each) for 20 h. Results of three independent experiments are presented. Cells from the same donor are linked. Values are means of duplicate samples. Statistical difference was evaluated by paired t-test. (c) Granzyme B level in freshly isolated HLA-DR-positive and negative NK cells. Values are mean ± SD of three independent experiments. Statistical difference was evaluated by paired t-test. (d, e) Antibody-dependent (d) and natural (e) cytotoxicity of four NK cell subsets toward C1R and K562 cells, respectively, measured after 20 h incubation with IL-2 (500 U mL−1). Values are mean ± SEM of three independent experiments. Statistical difference was evaluated by paired t-test. n/stim — nonstimulated, Rm — Rituximab (anti-CD20 antibody), C1R 56br — CD56bright cells + C1R cells, C1R 56dim — CD56dim cells + C1R cells; experimental samples contained NK cells of indicated subset +C1R cells +Rituximab.
As for cytotoxic activity, CD56dimHLA-DR+ NK cells were less effective in antibody-dependent killing than their HLA-DR− counterparts, although granzyme B level was equal between HLA-DR-positive and negative cells (Figure 2c and d); CD56bright cells both HLA-DR+ and HLA-DR− had low level of degranulation (Figure 2d). Natural cytotoxicity levels after overnight incubation with IL-2 (500 U mL−1) were similar among all four subsets: CD56brightHLA-DR−, CD56brightHLA-DR+, CD56dimHLA-DR−, CD56dimHLA-DR+ (Figure 2e).
Thus, among CD56dim NK cells, HLA-DR-positive cells show increased IFN-γ production but weaker degranulation than HLA-DR-negative cells in response to cytokine stimulation. CD56bright HLA-DR+ and HLA-DR− NK cells behaved according to typical characteristics of CD56bright subset.
K562-mbIL21cells in combination with IL-2 stimulate an increase in HLA-DR expression in NK cells in vitro
To study changes in HLA-DR expression during prolonged cultivation of NK cells, which might be required when preparing cells with certain characteristics for clinical use, we analyzed HLA-DR surface expression in NK cells stimulated with the following factors: IL-2, the combination of IL-2 and K562-mbIL21 cells, the combination of IL-2 and unmodified K562 cells, or K562-mbIL21 cells alone. Data on the unmodified K562 cells alone are not presented, as these cells were not able to maintain the viability of NK cells for more than 2–3 days. K562 feeder cells expressing IL-21 are successfully used for long-term cultivation and expansion of human NK cells.24 We observed that in the presence of IL-2 and/or K562-mbIL21 cells proportion of HLA-DR-positive NK cells in all groups of samples substantially increased after the 6th day of culture (Figure 3a and b). The size and granularity of the NK cells increased too, they became “irregular” in shape characterizing motile cells, and gathered in clearly distinguishable clusters, which altogether indicated that they passed into activated state. Cells in control samples (w/o stimulus) lived no more than 4–5 days, and their surface expression of HLA-DR did not increase (data not shown). Among samples analyzed, the highest HLA-DR levels were observed on day 6 and 9 in NK cells stimulated with the combination of IL-2 and K562-mbIL21 (35–93% HLA-DR+ cells depending on the donor) or with K562-mbIL21 only (47–90% HLA-DR+ cells) (Figure 3a and b). At the same time, the level of HLA-DR-positive NK cells on day 6 in the samples with IL-2 and unmodified K562 was also rather high (12–55%), which indicates that not only mbIL-21, but also other molecules on the surface of K562 cells play role in the increase of HLA-DR-positive NK cells proportion.
Figure 3.
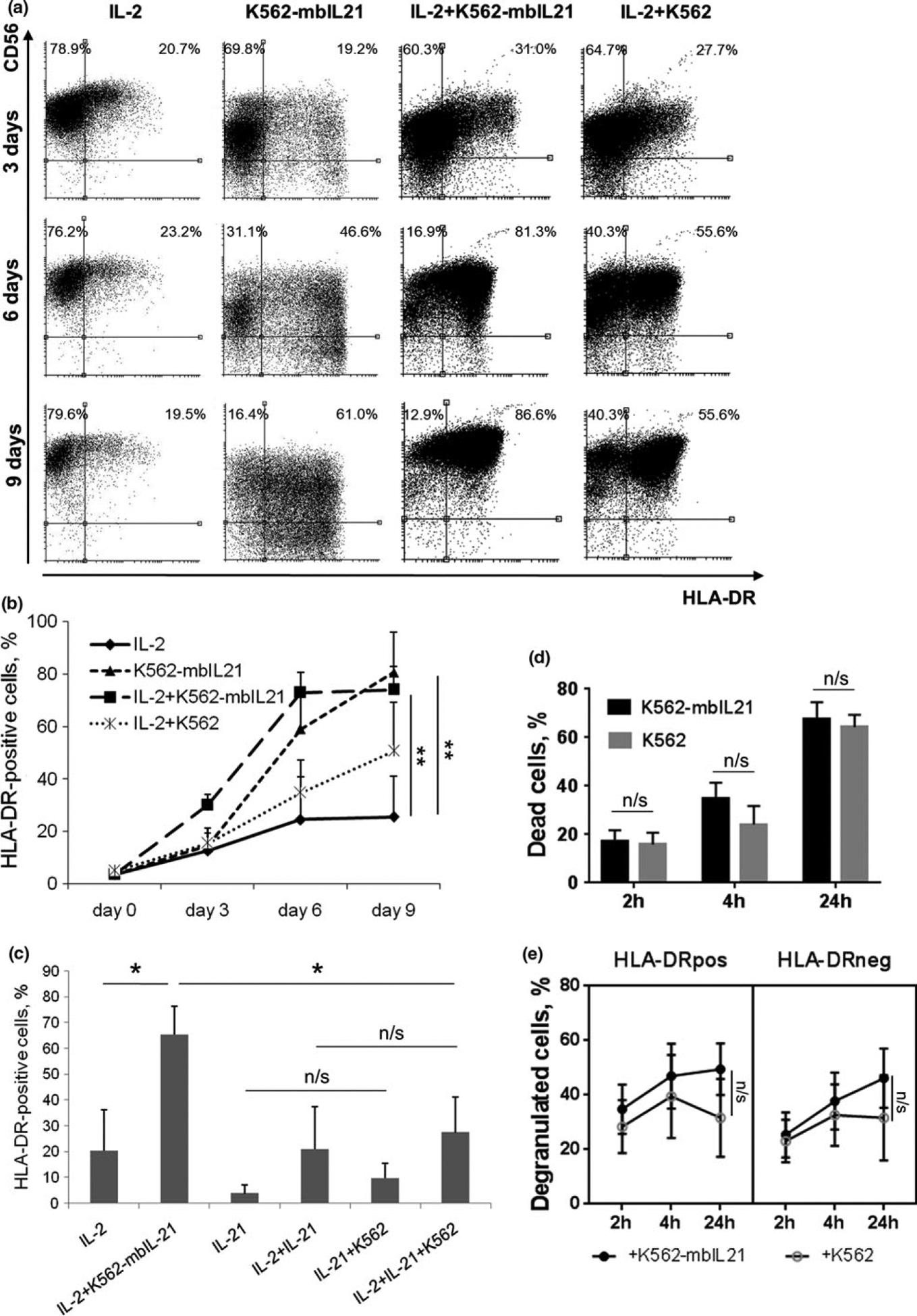
Increase in HLA-DR expression in NK cells upon stimulation with IL-2 and/or K562-mbIL21. (a) HLA-DR and CD56 expression on NK cells after 3, 6 and 9 days of incubation with indicated stimuli. Representative staining of cells from one donor out of three donors examined is shown. (b) Dynamics of HLA-DR expression during incubation with indicated stimuli. Values are mean ± SD of three independent experiments. Statistical difference, evaluated by paired t-test, is shown for day 9 time point. (c) Comparison of effects of soluble and membrane-bound IL-21 on HLA-DR surface expression in NK cells analyzed on the 6th day of incubation with indicated stimuli. Values are mean ± SD of three independent experiments. Statistical difference was evaluated by paired t-test. (d) Death rate of feeder cells of K562 or K561-mbIL21 cell lines after 2, 4 and 24 h of coincubation with NK cells. Cell death was measured with SytoxRed live/dead stain. Values are mean ± SD of three independent experiments. Statistical difference between groups was evaluated by paired t-test. (e) Degranulation of HLA-DR-positive and negative NK cells after 2, 4 and 24 h of coincubation with K562 or K562-mbIL21 feeder cells. Values are mean ± SEM of three independent experiments. Statistical difference between groups was evaluated by paired t-test at 24 h time point.
It should be noted that the number of living cells in NK cell samples stimulated with K562-mbIL21 alone on the 6th and especially on the 9th day was significantly lower than in the other samples, the cells were losing CD56 expression and dying out gradually (Figure 3a). Therefore, in this case the high percentage of HLA-DR-positive cells could be explained by preferential survival and proliferation of the activated HLA-DR+cells. In samples stimulated with IL-2 and K562-mbIL21 highly positive HLA-DR+ NK cells were also losing CD56 expression, perhaps, dying due to hyperactivation or excessive degranulation toward feeder cells. Nevertheless, NK cells in these samples displayed the greatest increase in the proportion of HLA-DR-positive cells in this stimulation model.
Next, we also compared the effects of soluble and membrane-bound IL-21 on HLA-DR-positive NK cell proportion. Soluble IL-21 with or without IL-2 did not increase significantly the level of HLA-DR+ NK cells compared to IL-2 alone (Figure 3c). Thus, the presence of feeder cells, expressing membrane-bound IL-21, plays a crucial role in the process. When the stimulation with soluble IL-2 was intensified by unmodified K562 cells, there was a tendency of HLA-DR-positive NK cells level increase, but it was nonsignificant. Apparently, cell-to-cell interactions involving other molecules on the surface of K562 feeder cells take certain part in NK cell activation, as it was already mentioned earlier, but to a lesser extent than membrane-bound IL-21.
Considering possible reasons of the up-regulating influence of K562-mbIL21 feeder cells on HLA-DR-positive NK cell proportion, we hypothesized that unmodified K562 cells and K562-mbIL21 cells may induce different cytotoxic response in NK cells, so that more or less NK cells undergo degranulation and further activation (including induction of HLA-DR expression or preferable HLA-DR+ cells proliferation). However, there appeared to be no difference neither in K562 versus K562-mbIL21 cells lysis, nor in NK cell degranulation toward these two cell lines (among both HLA-DR-positive and HLA-DR-negative NK cells) (Figure 3d and e).
In addition to HLA-DR+ NK cell subset increase, CD56 expression was rising during NK cell stimulation with IL-2. Most of the HLA-DR-positive cells demonstrated CD56bright phenotype after 6 days of cultivation and further (Figure 3a). Increase in CD56 expression in NK cells stimulated with different cytokines and feeder cells has been observed earlier.29,30 In samples containing only K562-mbIL21, CD56 expression was declining by the 9th day, which correlated with gradual extinction of the population (Figure 3a).
Stimulation with IL-2 and/or mbIL-21 induces both the expression of HLA-DR in HLA-DR-negative NK cells and HLA-DR-positive NK cell proliferation
Our next task was to verify whether the increase in HLA-DR-positive NK cell proportion happens due to a proliferation of sensitive to IL-2/mbIL-21 HLA-DR+ subpopulation, as shown by Evans et al., 2011, or whether the cytokine stimulation induces expression of HLA-DR in NK cells de novo. Results of this part of the study are presented in Figure 4a–d. Firstly, HLA-DR α-subunit gene expression was analyzed in NK cells by semiquantitative RT-PCR before and after the stimulation with IL-2 ± K562-mbIL21. In freshly isolated NK cells, the level of HLA-DR mRNA was rather low, but it increased after 6 days of stimulation (Figure 4b). Upon that, HLA-DR α-subunit gene expression was significantly higher when the combination of IL-2 and K562-mbIL21 was used for NK cell stimulation, compared to IL-2 alone.
Figure 4.
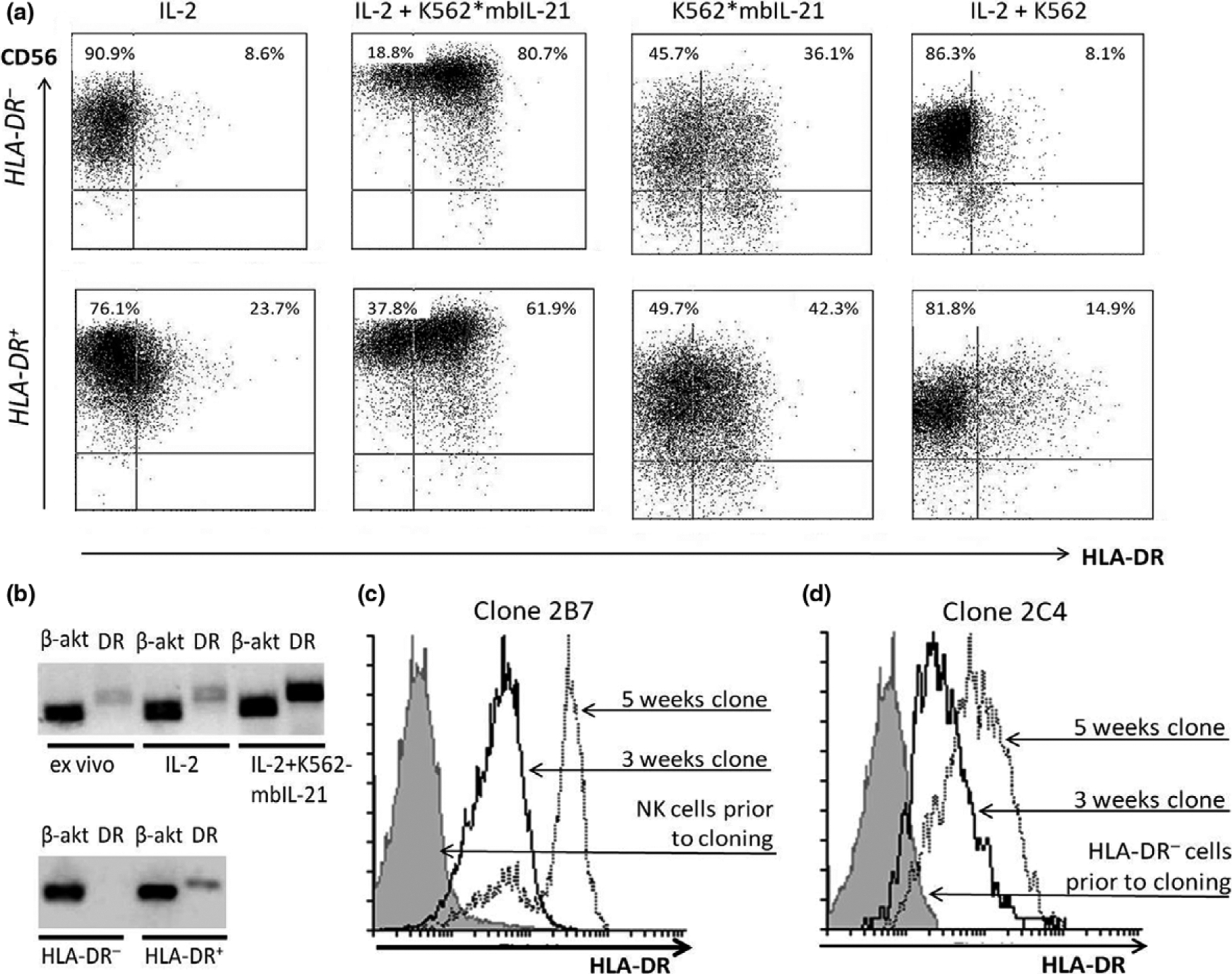
NK cell stimulation with IL-2 and/or K562-mbIL21 triggers HLA-DR surface expression on HLA-DR-negative cells. (a) HLA-DR expression in HLA-DR+ and HLA-DR− NK cell subsets after 6 days of incubation with indicated stimuli. (b) Presence of HLA-DR α-subunit mRNA in NK cells, freshly isolated and after 6 days of stimulation (upper row), and in HLA-DR-positive and HLA-DR-negative NK cells directly after sorting in “Single cell” mode (bottom row). (a, b) Representative data obtained from a single donor out of three donors examined are shown. (c) Changes in HLA-DR expression in clones, obtained from freshly isolated unsorted NK cells. (d) Changes in HLA-DR expression in clones, obtained from freshly isolated HLA-DR-negative NK cells subset. Representative data obtained from a single clone out of 20 (c) or 24 (d) clones examined, respectively, are shown in each figure.
On the next step we analyzed changes in HLA-DR expression induced by stimulation of HLA-DR− and HLA-DR+ cells obtained from freshly isolated NK cells by cell sorting (method 2) (Supplementary figure 2b). Analysis of mRNA level of HLA-DR α-subunit gene directly after sorting confirmed that in the sorted HLA-DR− subpopulation expression of this gene was absent, whereas in HLA-DR+ subset the expression was clearly visible (Figure 4b).
HLA-DR surface expression was analyzed in the HLA-DR− and HLA-DR+ NK cell subsets cultivated in vitro for 6 days with IL-2, IL-2+K562-mbIL21, K562-mbIL21 or IL-2+unmodified K562 (Figure 4a). Data on the unmodified K562 cells alone are not presented, as these cells were not able to maintain the viability of NK cells, and there remained virtually no living cells in these samples by day 6.
HLA-DR-positive NK cells appeared in HLA-DR– subset under all kinds of stimulation, but the proportion of HLA-DR+ cells in samples was different (Figure 4a). Response of HLA-DR-negative NK cells to IL-2 depended on the donor: the proportion of HLA-DR+ NK cells after 6 days of stimulation varied from 6.1 to 40.9%. When NK cells were stimulated with IL-2 and K562-mbIL21, the level of HLA-DR+ cells detected on day 6 was again the highest compared to all other kinds of stimuli. Thus, we have shown that HLA-DR expression appeared in cells initially negative for this marker, so IL-2 and mbIL-21 are capable of initiation of the HLA-DR expression de novo.
Interestingly, in HLA-DR+ NK cell subset the HLA-DR expression level measured after 6 days of in vitro cultivation was lower than directly after sorting. Internalization of HLA-DR molecules cross-linked with anti-HLA-DR antibody used for the sorting may be, at least partly, the reason for the decrease. Besides, being already activated, HLA-DRhi cells may have become less responsive to the stimuli such as IL-2 and mbIL-21.
To further confirm the hypothesis of de novo HLA-DR expression on the surface of NK cells stimulated by cytokines, we have generated and characterized NK cell clones. We have shown that clones derived both from the total NK cells and from the CD56+HLA-DR− subset increased expression of HLA-DR on the third/fifth week of their growth, and in certain clones almost 100% of cells in a clone highly expressed this molecule (Figure 4c, d, Supplementary table 2), confirming that HLA-DR appears on cells de novo following stimulation.
At the same time, we have compared proliferation activity of HLA-DR-positive and HLA-DR-negative NK cells in different stimulating conditions: in the presence of IL-2, IL-21, K562-mbIL21 cells, IL-2+IL-21, IL-2+K562 (unmodified), IL-2+K562-mbIL21. After 7 days of incubation, the proportion of proliferating cells among HLA-DR-positive NK cells was high in response to all the stimuli, with extremely high levels in the samples with K562-mbIL21 cells alone or in combination with IL-2 (Figure 5a and b). HLA-DR-negative NK cells showed rather high proliferation in samples with IL-2 and IL-2+K562-mbIL21; in other samples proliferation was weak (K562-mbIL21, IL-2+IL-21, IL-2+K562) or absent (IL-21). However, under all stimulating conditions, proliferation rate of HLA-DR+ cells was either significantly higher than of HLA-DR− cells, or at least there was a tendency for that (Figure 5a and b). Thus, HLA-DR expression is highly associated with NK cell proliferation, especially in response to K562-mbIL21 feeder cells.
Figure 5.
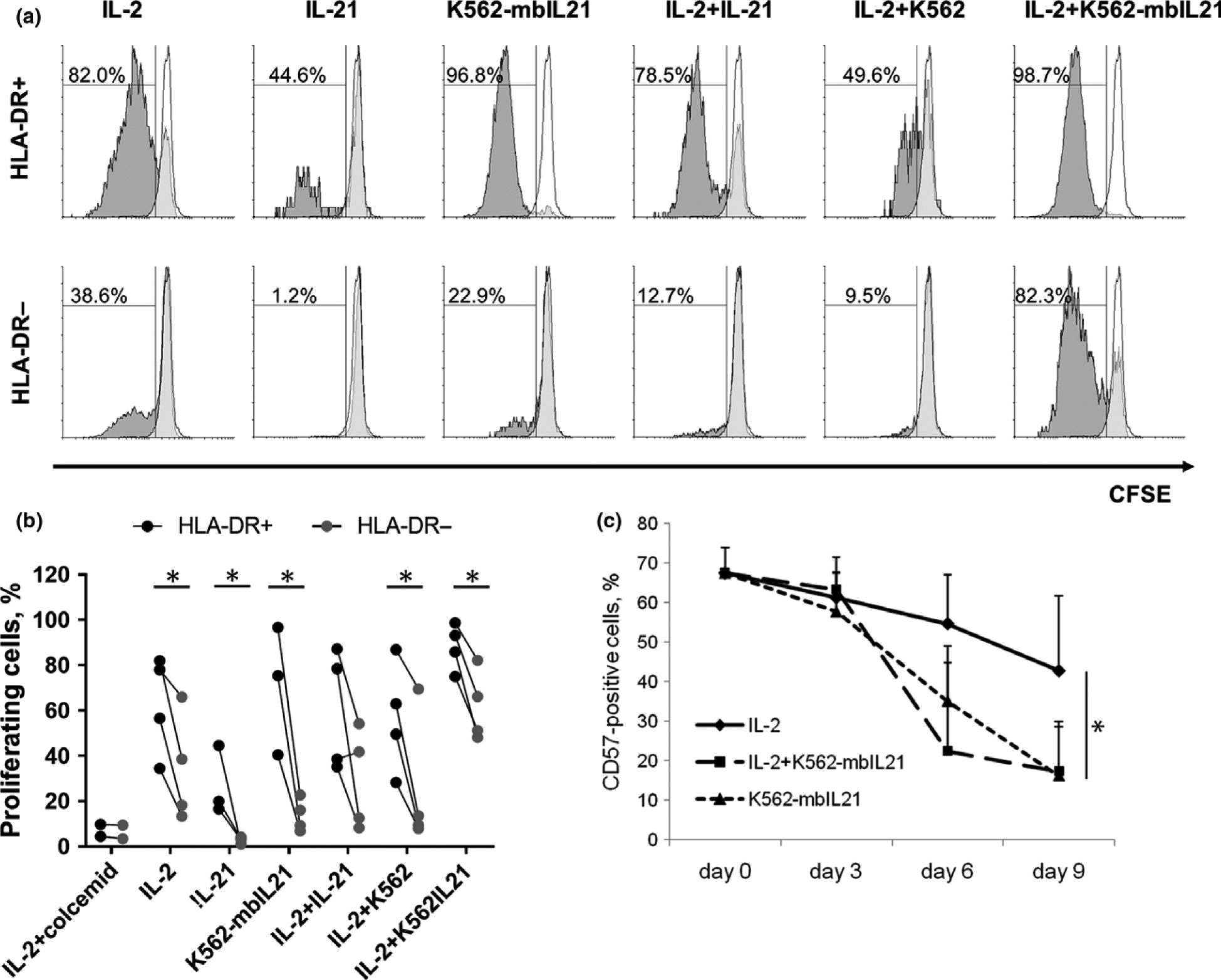
Proliferation and CD57 expression in NK cells upon stimulation with IL-2 and soluble or membrane-bound IL-21. (a, b) Proliferation of NK cells was estimated by loss of CFSE staining after 7 days of incubation with indicated stimuli. Representative staining (a) and general data of four independent experiments (b) are shown. (a) Overlay histograms show fluorescence intensity in control sample IL-2+colcemid (empty) and in the experimental samples (filled). (b) HLA-DR-positive and negative cells from the same donor are linked. Statistical difference was evaluated by paired t-test. (c) Dynamics of CD57 expression during incubation with indicated stimuli. Values are mean ± SD of three independent experiments. Statistical difference, evaluated by paired t-test, is shown for day 9 time point.
The highest proliferation rate in both HLA-DR-positive and HLA-DR-negative NK cells in the samples with IL-2+K562-mbIL21 is in accordance with the phenotype, acquired by NK cells during prolonged stimulation. We have found that the proportion of CD57+ NK cells decreased during cultivation with cytokines and feeder cells (Figure 5c), with the strongest effect observed in samples with IL-2+K562-mbIL21 and K562-mbIL21 only. As far as the appearance of CD57 on NK cell surface in the process of differentiation is considered to be irreversible,28 the cause of the effect should be preferential survival and proliferation of less differentiated CD57-negative cells in the presence of K562-mbIL21. Moreover, among this CD57− NK cell subset, 30–80% of the cells were HLA-DR-positive (data not shown). Presumably, the expression of HLA-DR during NK cell activation in vitro is associated mostly with CD57-negative cells, which proliferate in the first place in response to stimulation with IL-2 and mbIL-21.
To conclude, it can be argued that increased expression of HLA-DR in NK cells is a marker of both proliferation and certain state of NK cell activation.
Phenotypic features of HLA-DR-positive NK cells, obtained in culture
To reveal the special characters of HLA-DR-positive NK cells, we compared the expression of several phenotypic markers on HLA-DR+ and HLA-DR− NK cells ex vivo and after 3 or 6 days of cultivation with IL-2+K562-mbIL21, IL-2+K562 (unmodified) or IL-2+IL-21. Surface levels of LAMP-1 (CD107a), CD69, CD57, NKG2A, NKG2D and CD86 were measured. We found out that expression of CD69, CD57 and NKG2A was quite similar in both HLA-DR-positive and negative NK cells (Figure 6a and b; Supplementary figure 3). Expression of CD69, which characterizes cells in activated state,31 increased by day 6 in all cells with all kinds of stimuli. The proportion of NKG2A-positive cells on day 6 was relatively high in both types of NK cells with all stimuli, yet with the tendency toward higher values in samples with IL-2+K562-mbIL21. At the same time, the proportion of CD57-positive cells varied between samples with different stimuli: in samples with IL-2+K562-mbIL21 most of the cells, both HLA-DR+ and HLA-DR−, were CD57-negative; in other samples the level of CD57-positive cells was high and comparable to ex vivo values (Figure 6a and b). These data support the idea that stimulation with IL-2 and K562-mbIL21 cells leads to survival and proliferation of NK cells mainly at earlier stages of differentiation, which are characterized by CD57−NKG2A+ phenotype.28 On the other hand, such changes can happen due to phenotype switch, as it was shown that NKG2A can be acquired again under certain stimulation after it has been lost during differentiation.32 Soluble IL-21 or unmodified K562 cells did not provide the effect observed for K562-mbIL21 cells.
Figure 6.
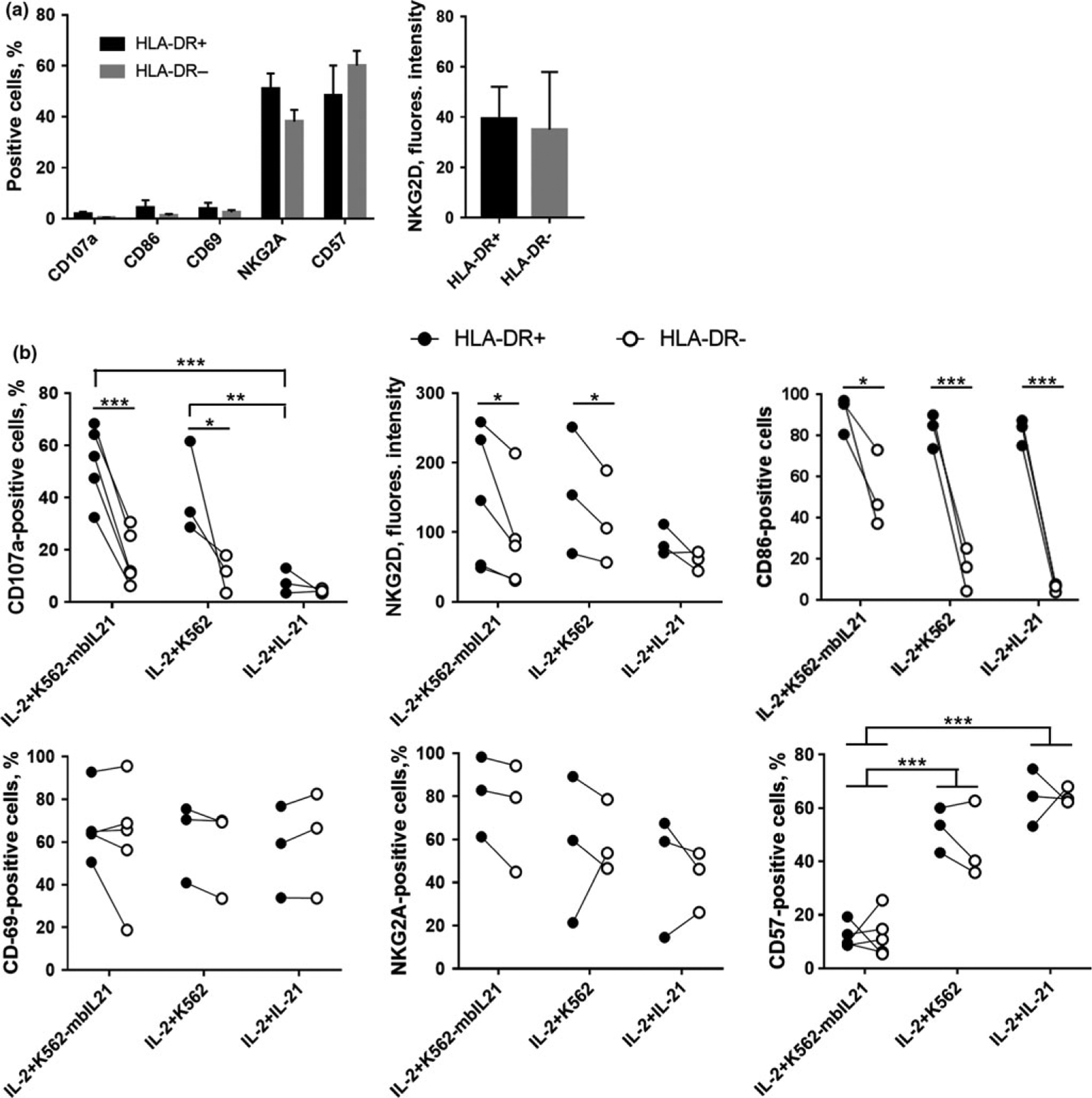
Surface markers expression in HLA-DR-positive and HLA-DR-negative NK cells. (a) Percentage of cells expressing LAMP-1, CD86, CD69, CD57, NKG2A, and expression intensity of NKG2D measured on day 0 in gated HLA-DR-positive and HLA-DR-negative NK cells. Values are mean ± SEM of minimum three independent experiments. (b) Percentage of cells expressing LAMP-1, CD86, CD69, CD57, NKG2A, and expression intensity of NKG2D measured after 3 or 6 day stimulation with IL-2+K562-mbIL-21, IL-2+K562 (unmodified) or IL-2+IL-21 in HLA-DR+ NK cells compared to HLA-DR− NK cells in all donors examined. Statistical difference between HLA-DR+ and HLA-DR− NK cells within one sample was evaluated by paired t-tests. Statistical difference between samples (different stimuli) was evaluated by two-way ANOVA multiple comparisons.
We established that expression of CD107a, NKG2D and CD86 differed between HLA-DR+ and HLA-DR− NK cells (Figure 6). HLA-DR-positive NK cells had higher proportion of CD107a+ cells on day 3 of stimulation compared to HLA-DR-negative NK cells in all samples which contained feeder cells. CD107a is the marker of NK cells degranulation, so, together with higher expression of activating receptor NKG2D in samples stimulated with IL-2+K562-mbIL21 or IL-2+K562, its presence shows that HLA-DR-positive NK cells obtained in these stimulation conditions demonstrated more intense natural cytotoxicity against both types of feeder cells. For CD107a marker, an earlier time point (day 3) was analyzed, as on day 6 the level of degranulated cells was very low in most of the donors. This could be caused by fewer feeder cells left by that time, which means fewer target cells. On the other hand, under prolonged stimulation NK cells may have acquired a kind of anergy, for example, due to granzyme B pool exhaustion.
A significant difference in the expression level between HLA-DR+ and HLA-DR− NK cells with all kinds of stimulation was also registered for CD86, costimulatory molecule required for T cell activation through the interaction of TCR and MHC molecules. The proportion of CD86-positive cells was highly increased in HLA-DR+ NK cells under all stimulating conditions. Thus, NK cells acquire instruments, which can be potentially used for antigen presentation.
Functional activity of HLA-DR+ and HLA-DR− NK cells, obtained in culture
Next, we have addressed to whether HLA-DR expression in NK cells, acquired in vitro, is somehow associated with their functional activity, as we have observed for ex vivo HLA-DR+ cells. Firstly, freshly isolated NK cells were subjected to IL-2 and K562-mbIL21 stimulation. On day 6 HLA-DR+ and HLA-DR− subsets were isolated by cell sorting (method 3, Supplementary figure 2c). This method allowed to separate two subsets with higher purity, than method 2, due to higher proportion of HLA-DR-positive cells in the activated NK cell population. Next, we compared functional activity of the HLA-DR-positive and HLA-DR-negative NK cells after restimulation with IL-2, IL-12+IL15, or C1R cells + anti-CD20 antibodies. IFN-γ production and cytotoxic activity were estimated.
HLA-DR-positive NK cells showed higher production of IFN-γ than HLA-DR− cells with all kinds of stimuli. The intensity of this effect varied in different donors (Figure 7a and b; Supplementary figure 4). In addition to the intracellular staining, IFN-γ concentration was measured in supernatants from the same samples by ELISA method. Together the results of the intra- and extracellular IFN-γ analysis showed that HLA-DR-positive NK cells were better producers of IFN-γ than HLA-DR-negative counterparts, in all donors examined. Under stimulation with C1R cells + anti-CD20 antibody (stimulation through CD16 receptor) both subsets demonstrated very low IFN-γ production.
Figure 7.
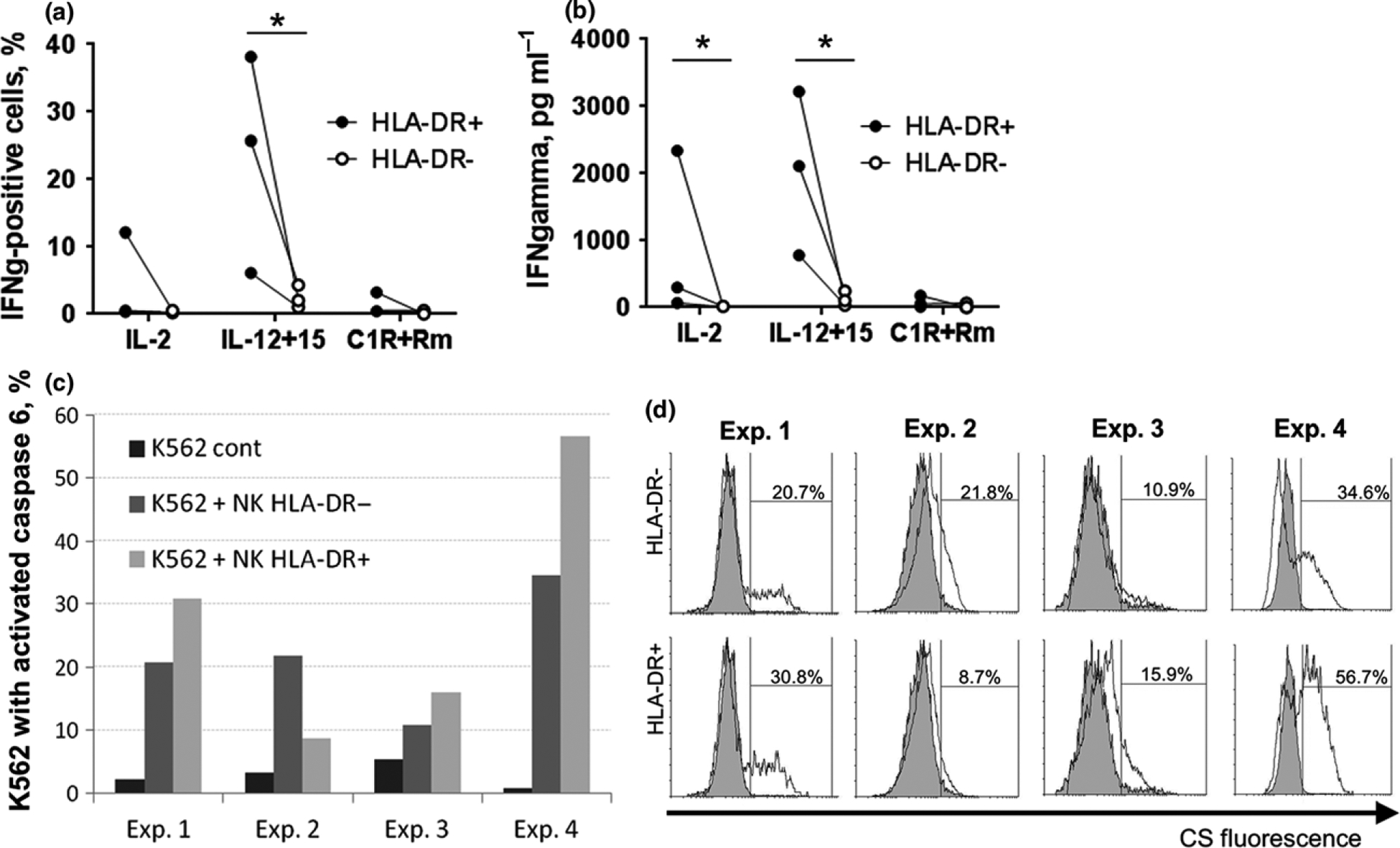
IFN-γ production and cytotoxic activity of HLA-DR-positive and HLA-DR-negative NK cells, sorted after 4 days of stimulation with IL-2 and K562-mbIL-21. (a, b) IFN-γ production of cells, cultivated for 24 h without stimuli and then restimulated overnight with IL-2, IL-15+IL-12, or with C1R cells and anti-CD20 antibodies, was measured by intracellular staining (a) and ELISA essay (b). Data of three independent experiments are shown. Cells from the same donor are linked. Values are mean of duplicate samples. Statistical difference was evaluated by paired t-test. (c, d) Cytotoxic activity of HLA-DR+ and HLA-DR− NK cells, evaluated according to caspase 6 substrate (CS) fluorescence in target K562 cells. Data obtained from four independent experiments are shown. (d) Overlay histograms show data acquired from K562 without effectors control sample (filled) and experimental sample (empty).
The results showing natural cytotoxic activity of the cells differed between donors (Figure 7c and d), but still HLA-DR+ NK cells showed a tendency to function more efficiently than HLA-DR− cells in three cases out of four. Low differences in the cytotoxicity level between HLA-DR+ and HLA-DR− subsets after 6 days of stimulation in certain donors may be caused by granzyme B pool exhaustion in HLA-DR-positive cells, as it was already mentioned before for CD107a expression.
Altogether, we can conclude that HLA-DR positive NK cells, obtained after 6 days of stimulation with IL-2 and K562-mbIL21, consist mostly of less differentiated CD56brightCD57−NKG2A+ cells, which display activated phenotype and more intense effector functions compared to HLA-DR-negative cells.
DISCUSSION
In healthy humans, HLA-DR-positive NK cells are commonly detected in small amounts in peripheral blood and spleen and in notably higher amounts in liver.9 HLA-DR expression is considered as a marker of NK cell activation and proliferation; the numbers of HLA-DR-positive NK cells in peripheral blood are elevated in a range of pathological conditions accompanied by inflammation.5,10–12 However, the significance of HLA-DR expression in a small pool of circulating NK cells in healthy individuals is poorly understood. In this work, we have analyzed more precisely HLA-DR expression in NK cell subsets, which differed by their maturity and functional characteristics. In our study, circulating HLA-DR+ NK cells comprised 0.8–22.1% of total NK cells in peripheral blood of healthy donors. Most of HLA-DR-positive cells displayed CD56bright phenotype, typical for less differentiated NK cells (Supplementary table 1, group 1). Interestingly, we have distinguished a group of individuals, who display much higher proportion of HLA-DR+CD56dimCD57+ NK cells compared to the others (supplementary table 1, group 2). Increased HLA-DR level in highly differentiated CD57+ cells corresponded to elevated HLA-DR expression in total NK cell population in most of these individuals. Possibly, various physiological situations involving transient or chronic inflammation may be associated with the increase in HLA-DR expression in these NK cells. Perhaps, part of HLA-DR+ NK cells, often referred to as cells in activated state,5 can survive for a long time and join the pool of “adaptive” NK cells, which are characterized, among other things, by high CD57 expression.33 HLA-DR expression analysis displayed in this paper represents snapshots of events associated with NK cell activation in these individuals.
In this work we also describe and discuss HLA-DR+ NK cells, generated in the stimulation model using IL-2 and K562 cells expressing membrane-bound IL-21. Both IL-2 and IL-21 activate NK cells; they induce proliferation of NK cells, increase their functional activity, and modify the repertoire of their surface receptors.4,16,30,34 Therefore, several models of NK cell expansion based on combinations of IL-2 and IL-21 (both soluble- and membrane-bound) are now considered for clinical use.16,24,25,35 We have shown that stimulation with a combination of IL-2 and K562-mbIL21 causes vast increase in HLA-DR-positive NK cell proportion, compared to IL-2 used separately: from 7% ex vivo (on average) up to 94% of all NK cells after 6 days of stimulation in vitro (Figure 3a and b). The influence of IL-2 on HLA-DR-positive NK cell subpopulation has been demonstrated in earlier work.13 At the same time, it was shown that soluble IL-21 alone does not increase the level of HLA-DR-positive NK cells,30 and our data are consistent with these findings (Figure 3c). However, when used together, IL-2 and IL-21 can increase the proportion of NK cells coexpressing CD86 and HLA-DR, as it has been shown recently by Loyon et al., 2016 and supported by us. Evans et al. state that increase in HLA-DR+ subpopulation occurs due to preferential proliferation of HLA-DR-positive cells in the presence of IL-2, but not due to HLA-DR expression on previously negative cells. They have demonstrated that stimulation of presorted HLA-DR− subset with IL-2 for 6 days increases the proportion of HLA-DR-positive cells to a very little extent, which was probably caused by contamination of HLA-DR− subpopulation with HLA-DR-positive cells during sorting process. In this study, we have also shown that stimulation of HLA-DR− subpopulation with IL-2 alone for 6 days leads to a minor increase in HLA-DR-positive cells proportion (less than 10% of NK cells) in most of the volunteers. However, there were individuals in which this proportion was significantly higher (up to 40.9%, data not shown). The situation was different when we used the combination of stimuli: IL-2 together with K562-mbIL21 caused an extremely high increase in HLA-DR-positive cells proportion in the presorted HLA-DR− subset (up to 80.7%) on the sixth day of the experiment. Thus, vast amounts of HLA-DR-positive cells appeared in initially negative subpopulation. So, we can state that HLA-DR+ NK cell expansion happens not only due to proliferation of preexisting HLA-DR-positive NK cells, but also due to induction of HLA-DR expression on NK cells de novo. This was confirmed by increased mRNA levels of HLA-DR α-subunit in NK cells activated by cytokines compared to resting NK cells, according to PCR results. Concerning the cases in which HLA-DR expression in presorted HLA-DR− subset increased only slightly in the presence of IL-2, the reason for such “low” sensitivity may be different amount of IL-2 receptors at the surface of NK cells in different donors. All NK cells express common γ-chain of IL-2 receptor, only small part of NK cells express α-chain, expression of β-chain may be decreased in certain terminally differentiated cells — all of that determines corresponding sensitivity to this cytokine.28
Still, we have confirmed earlier findings that HLA-DR-positive cells proliferate much more effectively in response to different cytokines and feeder cells than their HLA-DR-negative counterparts. It seems that HLA-DR-positive cells are extremely sensitive to IL-2 and K562-mbIL21 feeder cells (Figure 5a and b). Thus, increased expression of HLA-DR in NK cells is both a marker of proliferation and a certain “signal” acquired by NK cells during activation through cytokine stimulation and cell-to-cell contact.
There still remains a question why only a certain combination of stimuli induces HLA-DR expression in NK cells. The mechanism which activates MHC II (or its subtype HLA-DR) expression specifically in NK cells has not been described in literature yet. However, by analogy we can suggest that this process involves activation of transcription factor CIITA, either through its inducible promoter pIV (present in many cell types), or, due to proximity of NK cells to T cells by their origin and certain aspects of biology, through pIII promoter (present in B cells, T cells and dendritic cells). It is shown that induction of CIITA expression may be carried out through IFN-γ/STAT1-dependent pathway. It is known that STAT1 also takes part in signaling through IL-2 and IL-21 receptors.36,37 However, it was mentioned above that IL-21 alone does not influence the level of surface HLA-DR, thus, IL-21R downstream pathway does not influence CIITA gene activity. There are findings that IL-21 increases expression of CD25, common α-chain of IL-2 receptor.30 Our preliminary studies also show that incubation with K562-mbIL21 causes an upregulation of CD25 in NK cells (data not shown), which can lead to higher sensitivity to IL-2, and, as a possible result, to higher expression of HLA-DR molecule in NK cells. However, when we added combination of soluble IL-21 and IL-2 to NK cells, HLA-DR expression increased only slightly (Figure 3d); K562 feeder cells with membrane-bound IL-21 combined with IL-2 were much more effective. Thus, another mechanism apart from CD25-dependent IL-2R-STAT1 pathway is involved in CIITA activation; perhaps, other STAT factors are involved. It seems that cell-to-cell interactions between NK cells and feeder cells play a certain role in induction of HLA-DR expression: in samples with IL-2+unmodified K562 increase in HLA-DR-positive cell proportion was quite high (Figure 3a and b). First of all, interactions through activating NKG2D receptor might be involved, as K562 cells express appropriate ligands. Another reason might be expression of costimulatory molecule CD86 by K562 cells: in one of the studies it was demonstrated that NK cells have a variant of CD28 receptor recognizing CD86, and their interaction leads to NK cells transition into activated state.38,39 All in all, it is clear that HLA-DR expression does not accompany NK cell activation process in a broad sense, like, for example, CD69 marker does. It is most likely connected to intracellular activation pathways, induced by one kind of stimuli (cytokine or cell-to-cell interactions) but not by the other. We should keep in mind that stimuli which promote HLA-DR expression in NK cells in vivo, especially in CD56bright cells, might be different from IL-2 and mbIL21 used in our NK cell activation model in vitro. Nevertheless, at least in part our model is consistent with the situation that takes place in vivo, as expansion of HLA-DR+ NK cells during inflammatory response might be mediated by IL-21 produced by activated CD4+ T cells.
It should be noted that another possible reason for a significant increase in the proportion of HLA-DR-positive NK cells after incubation with K562-mbIL21 and IL-2 could be a phenomenon described as “trogocytosis” — “transfer” of part of the cell membrane from one cell to another when they are closely together. In particular this phenomenon has been described for NK cells interacting with dendritic cells.40 However, we have found that neither conventional K562 cells nor K562-mbIL21 cells do not express HLA-DR on their surface (data not shown). Thus, it seems that trogocytosis do not take part in our stimulation model.
Since we observed that ex vivo HLA-DR+ NK cells may accumulate mainly in two subpopulations, in less differentiated CD56bright cells and sometimes in more differentiated CD56dimCD57+ cells, we addressed the question whether HLA-DR+ NK cells generated during IL-2/K562-mbIL21 stimulation have a phenotypic similarity to any of these subsets. It has been shown earlier that IL-21 in combination with IL-2 increases expression of such markers as NKG2A, CD25, CD86 and CD69 in NK cells.30 In our study, activation of NK cells with IL-2 and K562-mbIL21 led to an elevation of the surface expression of NKG2A and decline of CD57+ cell proportion, so the resulting NK cells had less differentiated phenotype (Figure 6b). The resemblance of the activated NK cells to freshly isolated CD56bright NK cells was deepened by the increase on the CD56 expression level in NK cells after 6 days of stimulation with IL-2 alone and with combination of IL-2 and K562-mbIL21 (Figure 3a). Upregulation of CD56 has been shown earlier during prolonged multiday stimulation of NK cells with carcinoma cells coexpressing 4–1BBL and IL-12; at that NK cells changed their phenotype from CD56dim to CD56bright.29 The phenomenon was called phenotype “switching,” to point out that this is not a short-term increase in CD56 expression upon activation. Our results are consistent with this hypothesis, as high level of CD56 expression maintained even on the 12th day of stimulation. Furthermore, we have found that in samples with IL-2 and, to a much greater extent, in samples with IL-2 and K562-mbIL21 the proportion of CD57-positive NK cells significantly decreased during cultivation (Figure 3b). CD57− phenotype is thought to be typical for CD56bright cells. On the 6th day of stimulation up to 86% of NK cells were CD56brightCD57− (data not shown), and most of the HLA-DR-positive NK cells were detected among these type of cells. Up to now, there are several hypotheses describing CD56bright NK cell origin. The first one states that they are a stage in NK cell linear development between immature CD56neg and mature CD56dim NK cells.27,41 The others imply distinct origin of CD56bright cells, as an independent NK cell line derived from myeloid cells42 or from another lymphoid precursor.43 Thus, in our study the combination of IL-2 and mbIL-21 can induce proliferation of this CD56brightCD57− NK cell line, or it can cause “de-differentiation” of CD56dim cells. The last hypothesis has an indirect confirmation from the work of Skak, Frederiksen, and Lundsgaard (2008)30 as they have shown that the rise of CD56bright cells proportion under IL-2+IL-21 stimulation happened due to enhanced CD56 expression in CD56dim cells. We can imagine that certain number of such activated CD56bright NK cells, which used to be CD56dim, may contribute to CD56bright cell pool observed ex vivo.
To characterize more deeply functional characteristics of HLA-DR-expressing NK cells, we have compared functional activity of ex vivo HLA-DR+ and HLA-DR− NK cells from CD56bright and CD56dim subsets, as well as of in vitro IL-2/K562-mbIL21-stimulated HLA-DR+ and HLA-DR– NK cells. Both ex vivo and IL-2/K562-mbIL21-stimulated HLA-DR+ NK cells demonstrated higher IFN-γ response to IL-12/IL-15 compared to respective HLA-DR− cells. We have shown earlier that IFN-γ production in NK cells in response to IL-2 decreases gradually along with NK cell differentiation.44 In this work, cytokine-induced IFN-γ production in ex vivo CD56dim NK cells was also lower than in CD56bright cells. However, response of HLA-DR+CD56dim NK cells differed from that of HLA-DR−CD56dim cells in all performed experiments (Figure 2a and b).
In literature, there is an evidence that combination of IL-21 and IL-2 stimulates NK cells cytotoxic activity and production of IFN-γ.30 Also, it has been shown that freshly isolated peripheral blood HLA-DR+ NK cells exhibit enhanced cytotoxic activity against NK-resistant Daudi cell line compared to HLA-DR− NK cells from the same donor, but their cytotoxicity toward K562 cells did not differ.9 We have compared cytotoxic activity of the HLA-DR-positive and negative NK cells from the same donor, freshly isolated and sorted after 6 days of stimulation with IL-2 and K562-mbIL21. Ex vivo CD56dim HLA-DR+ NK cells demonstrated increased IFN-γ production but weaker antibody-dependent degranulation than HLA-DR-negative cells in response to cytokine stimulation. Natural cytotoxicity of freshly isolated HLA-DR-positive and negative NK cells was the same, in accordance to previous findings.
Concerning cells sorted after 6 days of stimulation with IL-2/K562-mbIL21, we observed that HLA-DR+ NK cells showed a tendency to more efficient natural cytotoxicity than HLA-DR− cells. Weak responses to stimulation through ADCC receptor in both HLA-DR+ and HLA-DR− NK cells suggest the lack of NK cells with adaptive features in IL-2/K562-mbIL21 stimulated NK cell cultures. Interesting feature of IL-2/K562-mbIL21 stimulated HLA-DR+ NK cells is an increased CD86 surface level. Together with HLA-DR, it might be considered as potential antigen-presenting capacity of these cells. Some evidences to date demonstrate that NK cells possess an ability to present certain antigens through HLA-DR molecule.9,14 On the other hand, we cannot firmly state that HLA-DR does not function as a ligand for an unknown receptor itself. Potential interactions of these cells with T cells or CD4+ monocytes should be studied further in more detail.
Thus, after 6-day activation with the cytokines, HLA-DR+ NK cells demonstrated pronounced differences in phenotype and function from HLA-DR− NK cells. Due to their special features, such as increased IFN-γ production in response to cytokines and a potential for antigen presentation, HLA-DR+ NK cells obtained in vitro seem a promising agent for therapeutic use. However, it should be noted, that physiological significance of these HLA-DR-positive NK cells in humans as a marker of chronic immune activation is still under discussion.10 Possible side effects (including inhibiting interactions with T cells) or loss of functional activity of in vitro activated HLA-DR+ NK cells after introduction in vivo should be taken into account and need more profound study.
METHODS
Cell lines
K562 and C1R cell lines were obtained from ATCC (Manassas, VA, USA). The genetically modified clone of K562 cells expressing membrane-bound IL-21 (К562-mbIL21) was kindly provided by Dr Dean Lee (MD Anderson Cancer Center, USA). This clone is characterized as tCD19+CD64+CD86+CD137L+. All cell lines were cultivated in the following culture medium: RPMI-1640 (PanEco, Russia) supported with 10% FCS (fetal calf serum) (HyClone, USA), 2 mmol L−1 of l-glutamine (PanEco) and Antibiotic Antimycotic Solution (Sigma-Aldrich, St. Louis, MO, USA). Surface expression of IL-21 was tested periodically by flow cytometry. K562-mbIL21 cells and unmodified K562 cells were irradiated with γ radiation (100 Gy), cryopreserved at −150°C and then recovered prior to NK cell stimulation.
Isolation of human NK cells
This study was carried out in accordance with the recommendations of local ethics committee in accordance with the Declaration of Helsinki. Blood samples were obtained from healthy volunteers of different age and sex (30 persons), who gave written informed consent prior to the study. Peripheral blood mononuclear cells (PBMC) fraction was obtained by gradient centrifugation using a standard Ficoll solution (PanEco), density 1.077. NK cells were isolated from PBMC using NK cell isolation kit (Miltenyi Biotec, Germany) by negative magnetic separation, according to the manufacturer’s protocol. NK cells purity was checked by flow cytometry for compliance with CD3−CD14−CD20−CD56+ phenotype. In addition, admixture of CD56+CD7− cells was analyzed.
Flow cytometry
Cells were labeled with mouse anti-human monoclonal antibodies (mAb) and analyzed as described by Kovalenko et al., 2017.45 The following mAbs were used: CD56-APC, clone N901; CD56-PE, clone N901; CD3-FITC, clone UCHT1; HLA-DR-FITC, clone Immu-357 (Beckman Coulter, Miami, FL, USA); CD16-PE, clone 3G8; CD14-PE, clone M5E2; CD3-APC, clone UCHT1 (BD Biosciences, San Jose CA, USA); CD57-PE, clone TB01; CD69-PE, clone FN50; LAMP-1-FITC, clone eBioH4A3; CD107a (LAMP-1)-PE-Cy5, clone eBioH4A3; NKG2D-PE, clone 1D11 (eBioscience, San Diego, CA, USA); CD3-PE, clone UCHT1 (Dako, Denmark); NKG2A-PE, clone REA110; CD57-APC, clone TB03 (Miltenyi Biotec); CD86-PE, clone IT2.2; IL-21-PE, clone 3A3-N2 (BioLegend, USA); HLA-DR-PE, HLA-DR-Brilliant Violet 421, clone L243; CD56-PE-Cy7, clone 5.1H11 (Sony Biotechnology, USA). SytoxRed live/dead stain was used to exclude dead NK cells and evaluate K562/K562-mbIL21 cell death. Cytometric analysis of samples was performed on a FACSCalibur flow cytometer (BD Biosciences) equipped with 488- and 640-nm lasers. Lymphocytes were gated according to forward and side scatter. NK cells were gated according to CD56 expression, and at least 20 000 events were recorded in this gate for the analysis of NK cells isolated by magnetic separation and at least 5000 events for the analysis of NK cell clones and sorted subpopulations. Data were analyzed using Flowing Software (Turku Centre for Biotechnology, Finland).
NK cell sorting
Freshly isolated NK cells (in method 1 and 2, Supplementary figure 2a and b) or NK cells, prestimulated for 6 days with IL-2 (Hoffmann-La-Roche, Switzerland) and K562-mbIL21 (in method 3, Supplementary figure 2c), were stained with antibodies to CD56, CD3, HLA-DR as described above, then subjected to sterile fluorescence-activated sorting. FACSVantage DiVa cell sorter (BD Biosciences) equipped with 405-, 488- and 643-nm lasers with dichroics and filters appropriate for the above fluorochromes was used. Cells were sorted into 12 × 75 mm tubes or 96-well U-bottom plates. In case of ex vivo NK cells (method 1 and 2) we managed to obtain only enriched subpopulations, as HLA-DR-positive cells comprise a very small amount of all peripheral NK cells. Sorted subpopulations were transferred into culture medium and used in further experiments.
Proliferation assay
Proliferation of NK cells was estimated by CFSE test. Freshly isolated NK cells were stained with 5 μmol L−1 mL−1 of CFSE (eBioscience), then washed and transferred into the culture medium with the appropriate stimuli according to the experiment. NK cells supported with IL-2 (100 U mL−1) and colcemid (200 ng mL−1) were used as nonproliferating negative control. The percentage of cells losing CFSE staining was measured by flow cytometry on day 4 and 7. The medium was replaced on day 4.
Generation of NK cell clones
NK cells were stained as for cell sorting, then sorted into 96-well round-bottom plates, one cell per well in single cell sorting mode, according to the following phenotypes: CD3−CD56+, CD3−CD56+HLA-DR+, CD3−CD56+HLA-DR−. Plates prepared for NK cells collection contained 2×103 K562-mbIL21 feeder cells per well in 200 μL of complete medium for clones: a mixture of DMEM medium (PanEco) and ExVivo medium (Thermo Fisher Scientific, Carlsbad, CA, USA) at 4:1 ratio, supplemented with 10% FCS (HyClone), 2 mmol L−1 of L-glutamine (PanEco), Antibiotic Antimycotic Solution (Sigma-Aldrich) and 100 U mL−1 of recombinant human IL-2 (Hoffmann-La-Roche, Switzerland). Plates with sorted NK cells were incubated at 37°C, with 5% CO2 for 3 weeks or more. Every 3 weeks of incubation half of the medium was replaced. Surface expression of HLA-DR was measured at week 3 or 5 by flow cytometry.
NK cells multiday activation with cytokines and/or feeder cells
Freshly isolated NK cells or sorted NK cell subpopulations were transferred to culture medium and placed into 96-well or 24-well plates at the concentration of 106 cells per mL. IL-2 (100 U mL−1), IL-21 (10 ng mL−1, Biolegend), K562-mbIL21 cells (4:5 with NK cells) and unmodified K562 cells (4:5 with NK cells) in different combinations (see the corresponding figures) were added to NK cells. Cells were incubated at 37°C, with 5% CO2; half of the culture medium was replaced every 3 days. After 1, 3, 6, 9, or 12 days, depending on the experiment, NK cells were collected and analyzed.
NK cell stimulation for functional assays
To estimate functional responsiveness, NK cells were incubated for 20 h in culture medium, containing IL-2 (500 U mL−1), or IL-12 (10 ng mL−1; BD Biosciences) + IL-15 (10 ng mL−1; Sigma-Aldrich), or C1R cells + anti-CD20 antibody Rituximab (Roche, Switzerland). NK cells, preliminary cultivated with IL-2 and/or K562-mbIL21 and sorted into HLA-DR+ and HLA-DR− subsets, were transferred into new medium, then treated with the stimuli indicated above either directly after sorting, or 24 h later after a period of rest. At the end of the incubation, cells were harvested and subjected to functional assays.
Cytotoxicity assays
In freshly isolated NK cells, cytotoxicity was estimated by NK cell degranulation according to CD107a (LAMP-1) marker expression. NK cells were incubated with target cells (K562 or K562-mbIL21) at 2:1 ratio for 2, 4, or 24 h, depending on the experiment, in the presence of 10 μg mL−1 of Brefeldin A and anti-CD107a mAb (CD107a-PE-Cy5). After that cells were harvested and analyzed by flow cytometry.
In NK cells, preactivated with feeder cells, caspase-6 activation assay was used to measure death rate of K562 target cells, as NK cells already showed certain level of degranulation toward feeder cells. Active caspase-6 level in the target cells was estimated using the CyToxiLux PLUS Kit (OncoImmunin, USA), applied according to the manufacturer’s protocol. Briefly, K562 cells were labeled with TFL-4 dye and then added to NK cells in 1:1 ratio. After that, cells were pelleted, supernatants were replaced with 50 μL of caspase-6 specific substrate, and the samples were incubated for 30 min at 37°C. The percentage of K562 cells with activated caspase-6 was estimated using flow cytometry by the fluorescence of cleaved caspase 6 substrate.
Elisa
IFN-γ levels in supernatants, collected after incubation of NK cells under different conditions, were analyzed using IFN-γ ELISA kit (Vector-Best, Russia) as described previously.46 Optical density was measured using Multiscan FC plate reader (Thermo Fisher Scientific) with 450 nm basic filter and 620 nm reference filter.
Intracellular IFN-γ staining
For intracellular IFN-γ measurement 10 μg mL−1 of brefeldin A was added to NK cells 4 h prior to staining. NK cells were then transferred from the cell medium into PBS containing 0.5% BSA and 0.1% sodium azide and stained for surface markers as described above. Next, cells were fixed and permeabilized with CytoFix/CytoPerm solutions (BD Biosciences), washed two times with CytoPerm/CytoWash solution and incubated for 30 min at 4°C with IFN-γ-FITC mAb (Miltenyi Biotec). Isotype-matched mouse antibody (Miltenyi Biotec) was used as a negative control for the staining. Finally, cells were washed two times and analyzed by flow cytometry.
PCR analysis
Total RNA was extracted from NK cells after lysis in TRI-reagent (MRC, USA) using centrifugation with chloroform, then washed in isopropanol and 75% ethanol. cDNA was obtained using standard protocol for MMLV reverse transcriptase (Eurogen, Russia). PCR reaction was conducted using Taq-polymerase (Eurogen, Russia) under standard conditions. HLA-DR α-subunit primers were 5’ATCATGACAAAGCGCTCCAACTAT 3’, forward, 5’GATGCCCACCAGACCCACAG 3’, reverse. β-actin was used as a positive control.
Statistical analysis
Statistical significance of the differences in the data with normal distribution was determined by paired or unpaired Student’s t-tests. For non-normally distributed data the Mann–Whitney U-test was used. P < 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Dean Lee (MD Anderson Cancer Center, USA) for providing us with K562-mbIL21 cell line. This work was supported partly by Russian Foundation for Basic Research, grant #16-34-00836. NK cell ex vivo phenotypic and functional analysis, proliferation assay, cell sorting and NK cell clone experiments were supported by Russian Science Foundation, grant #16-15-00309.
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Shereck E, Satwani P, Morris E, Cairo MS. Human natural killer cells in health and disease. Pediatr Blood Cancer 2007; 49: 615–623. [DOI] [PubMed] [Google Scholar]
- 2.Caligiuri M Human natural killer cells. Blood 2008; 112: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol 2004; 22: 405–429. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Ye LJ, Ren HL, et al. Multiple effects of IL-21 on human NK cells in ex vivo expansion. Immunobiology 2015; 220: 876–888. [DOI] [PubMed] [Google Scholar]
- 5.Yano N, Endoh M, Nomoto Y, Sakai H, Rifai A. Increase of HLA-DR-positive natural killer cells in peripheral blood from patients with IgA nephropathy. Hum Immunol 1996; 49: 64–70. [DOI] [PubMed] [Google Scholar]
- 6.Ferlazzo G, Thomas D, Lin S-L, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol 2004; 172: 1455–1462. [DOI] [PubMed] [Google Scholar]
- 7.Galea-Lauri J, Darling D, Gan SU, et al. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol 1999; 163: 62–70. [PubMed] [Google Scholar]
- 8.Rock KL, Reits E, Neefjes J. Present yourself! by MHC class I and MHC class II molecules. Trends Immunol 2016; 37: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burt BM, Plitas G, Nguyen HM, Stableford JA, Bamboat ZM, Dematteo RP. Circulating HLA-DR+ natural killer cells have potent lytic ability and weak antigen-presenting cell function. Hum Immunol 2009; 69: 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogli M, Costa P, Murdaca G, et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol 2004; 34: 2313–2321. [DOI] [PubMed] [Google Scholar]
- 11.Lichtfuss GF, Cheng W-J, Farsakoglu Y, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol 2012; 189: 1491–1499. [DOI] [PubMed] [Google Scholar]
- 12.Aranami T, Miyake S, Yamamura T. Differential expression of CD11c by peripheral blood NK cells reflects temporal activity of multiple sclerosis. J Immunol 2006; 177: 5659–5667. [DOI] [PubMed] [Google Scholar]
- 13.Evans JH, Horowitz A, Mehrabi M, et al. A distinct subset of human NK cells expressing HLA-DR expand in response to IL-2 and can aid immune responses to BCG. Eur J Immunol 2011; 41: 1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncarolo MG, Bigler M, Haanen JB, et al. Natural killer cell clones can efficiently process and present protein antigens. J Immunol 1991; 147: 781–787. [PubMed] [Google Scholar]
- 15.Loyon R, Picard E, Mauvais O, et al. IL-21-induced MHC class II + NK cells promote the expansion of human uncommitted CD4 + central memory T cells in a macrophage migration inhibitory factor-dependent manner. J Immunol 2016; 197: 85–96. [DOI] [PubMed] [Google Scholar]
- 16.Park Y-K, Shin D-J, Cho D, et al. Interleukin-21 increases direct cytotoxicity and IFN-γ production of ex vivo expanded NK cells towards breast cancer cells. Anticancer Res 2012; 32: 839–846. [PubMed] [Google Scholar]
- 17.van Ostaijen-ten Dam MM, Prins H-J, Boerman GH, et al. Preparation of cytokine-activated NK cells for use in adoptive cell therapy in cancer patients. J Immunother 2016; 39: 90–100. [DOI] [PubMed] [Google Scholar]
- 18.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408: 57–63. [DOI] [PubMed] [Google Scholar]
- 19.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105: 3051–3057. [DOI] [PubMed] [Google Scholar]
- 20.Jiang B, Wu X, Li XN, et al. Expansion of NK cells by engineered K562 cells co-expressing 4–1BBL and mMICA, combined with soluble IL-21. Cell Immunol 2014; 290: 10–20. [DOI] [PubMed] [Google Scholar]
- 21.Suck G, Oei VYS, Linn YC, et al. Interleukin-15 supports generation of highly potent clinical-grade natural killer cells in long-term cultures for targeting hematological malignancies. Exp Hematol 2011; 39: 904–914. [DOI] [PubMed] [Google Scholar]
- 22.Fujisaki H, Kakuda H, Imai C, Mullighan C, Campana D. Replicative potential of human natural killer cells. Br J Haematol 2009; 145: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapteva N, Durett AG, Sun J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012; 14: 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012; 7: e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Wu H-W, Sheard MA, et al. Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive cell therapy. Clin Cancer Res 2013; 19: 2132–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan A, Hong D, Atzberger A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol 2007; 179: 89–94. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani C, Juelke K, Falco M, et al. CD56brightCD16-killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol 2007; 178: 4947–4955. [DOI] [PubMed] [Google Scholar]
- 28.Björkström NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010; 116: 3853–3864. [DOI] [PubMed] [Google Scholar]
- 29.Dowell AC, Oldham KA, Bhatt RI, Lee SP, Searle PF. Long-term proliferation of functional human NK cells, with conversion of CD56 dim NK cells to a CD56 bright phenotype, induced by carcinoma cells co-expressing 4–1BBL and IL-12. Cancer Immunol Immunother 2012; 61: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skak K, Frederiksen KS, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008; 123: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzio R, Mauël J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol 1999; 21: 565–582. [DOI] [PubMed] [Google Scholar]
- 32.Béziat V, Descours B, Parizot C, Debré P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS ONE 2010; 5: e11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “Memory”. J Immunol 2011; 186: 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunne J, Lynch S, O’Farrelly C, et al. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol 2001; 167: 3129–3138. [DOI] [PubMed] [Google Scholar]
- 35.Granzin M, Stojanovic A, Miller M, Childs R, Huppert V, Cerwenka A. Highly efficient IL-21 and feeder cell-driven ex vivo expansion of human NK cells with therapeutic activity in a xenograft mouse model of melanoma. Oncoimmunology 2016; 5: e1219007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habib T, Nelson A, Kaushansky K. IL-21: a novel IL-2–family lymphokine that modulates B, T, and natural killer cell responses. J Allergy Clin Immunol 2003; 112: 1033–1045. [DOI] [PubMed] [Google Scholar]
- 37.Delespine-Carmagnat M, Bouvier G, Bertoglio J. Association of STAT1, STAT3 and STAT5 proteins with the IL-2 receptor involves different subdomains of the IL-2 receptor β chain. Eur J Immunol 2000; 30: 59–68. [DOI] [PubMed] [Google Scholar]
- 38.Costa C, Barber DF, Fodor WL. Human NK cell-mediated cytotoxicity triggered by CD86 and Gal alpha 1,3-Gal is inhibited in genetically modified porcine cells. J Immunol 2002; 168: 3808–3816. [DOI] [PubMed] [Google Scholar]
- 39.Wilson JL, Charo J, Martín-Fontecha A, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immunol 1999; 163: 4207–4212. [PubMed] [Google Scholar]
- 40.Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci 2011; 108: 18360–18365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 2005; 22: 295–304. [DOI] [PubMed] [Google Scholar]
- 42.Mace EM, Hsu AP, Monaco-Shawver L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood 2013; 121: 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Li B, Lu R, et al. Clonal tracking of rhesus macaque hematopoiesis highlights a distinct lineage origin for natural killer cells. Cell Stem Cell 2014; 14: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanevskiy LM, Erokhina SA, Streltsova MA, et al. Bacterial lipopolysaccharide activates CD57-negative human NK cells. Biochemistry (Mosc) 2014; 79: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovalenko E, Streltsova M, Kanevskiy L, Erokhina S, Telford WG. Identification of human memory-like NK cells. Curr Protoc 79: 9.50.1–9.50.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanevskiy LM, Telford WG, Sapozhnikov AM, Kovalenko EI. Lipopolysaccharide induces IFN-γ production in human NK cells. Front Immunol 2013; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


