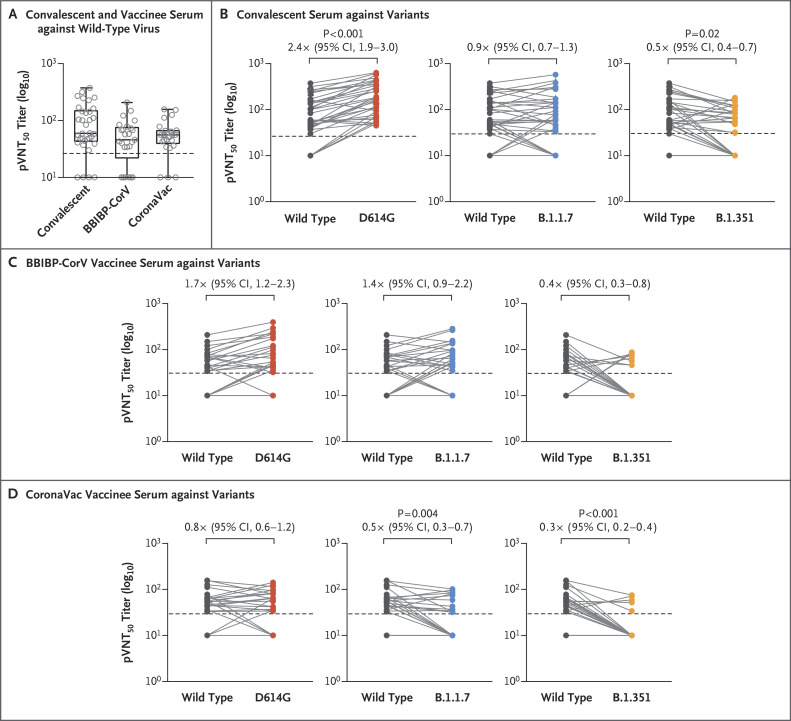
Figure 1. Neutralization of SARS-CoV-2 Pseudoviruses in Convalescent and Vaccinee Serum Samples.
Panel A shows the 50% pseudovirus neutralization titer (pVNT50) in convalescent serum collected from 34 recovered patients approximately 5 months after SARS-CoV-2 infection and in serum collected from 50 vaccinees who had received either the BBIBP-CorV or CoronaVac vaccine 2 to 3 weeks after the second dose against recombinant vesicular stomatitis virus–based SARS-CoV-2 pseudovirus bearing the Wuhan-1 (wild-type) spike protein. Box plots indicate the median and interquartile range (IQR); the whiskers represent 1.5 times the IQR. Panel B shows changes in the reciprocal serum pVNT50 titer in 34 convalescent serum samples against the D614G, B.1.1.7, and B.1.351 variants, as compared with wild-type virus. Panels C and D show changes in the reciprocal pVNT50 titer in serum samples obtained from the 25 recipients of the BBIBP-CorV vaccine and 25 recipients of the CoronaVac vaccine, respectively, against the D614G, B.1.1.7, and B.1.351 variants, as compared with wild-type virus. Factor changes in the geometric mean titer and 95% confidence interval (CI) in the pVNT50 titers, as compared with those for wild-type virus, are shown under the P values. Only P values of less than 0.05 (indicating significance) are shown. Each data point is the average of duplicate assay results. In each panel, the horizontal dashed line represents the lower limit of detection of the assay (titer, <30); this limit was assigned a value of 10 for geometric mean calculations and was considered to be seronegative. In all panels, calculations were performed with the use of the two-tailed Kruskal–Wallis test after adjustment for the false discovery rate.