Figure 1:
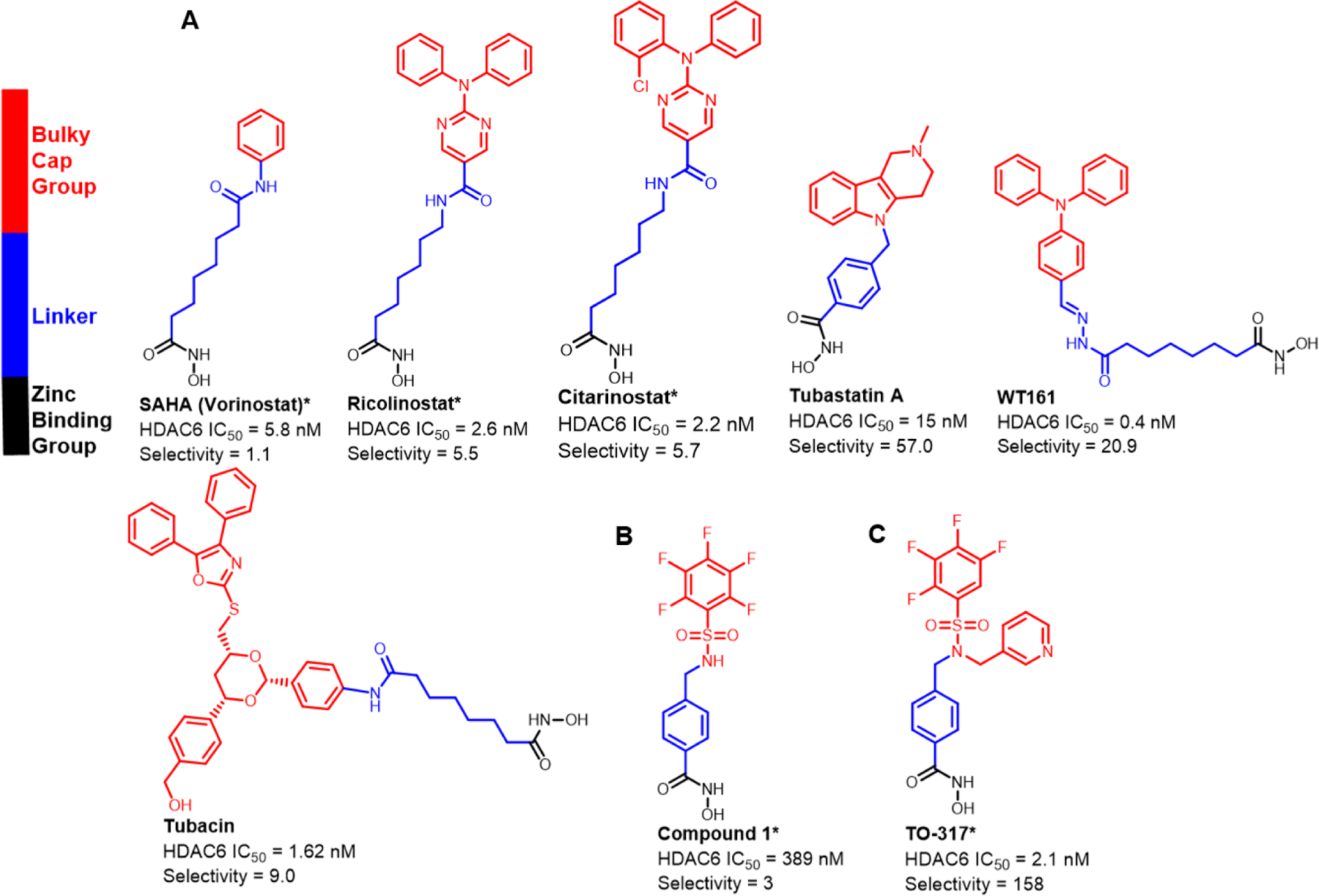
Currently known HDAC6 inhibitors. The structural features involve a hydroxamic acid which is required for Zn2+ binding, and a linker that connects the hydroxamic acid to a cap group. a) Currently known inhibitors achieved HDAC6 selectivity by replacing the benzene substituent on SAHA with a bulkier, usually hydrophobic cap group. b) Previous studies showed compound 1 is a nM inhibitor against HDAC6 exhibiting limited selectivity c) Current studies show TO-317 adopts a rotatable cap group with two aromatic substituents that occupy the HDAC6 surface facilitating specific residue interactions.
*IC50 values were determined using an activity-based electrophoretic mobility shift assay (EMSA) by Nanosyn Inc., USA.
