Abstract
Background
Malaria is highly heterogeneous: its changing malaria microepidemiology needs to be addressed to support malaria elimination efforts at the regional level.
Methods
A 3-year, population-based cohort study in 2 settings in the Peruvian Amazon (Lupuna, Cahuide) followed participants by passive and active case detection from January 2013 to December 2015. Incidence and prevalence rates were estimated using microscopy and polymerase chain reaction (PCR).
Results
Lupuna registered 1828 infections (1708 Plasmodium vivax, 120 Plasmodium falciparum; incidence was 80.7 infections/100 person-years (95% confidence interval [CI] , 77.1–84.5). Cahuide detected 1046 infections (1024 P vivax, 20 P falciparum, 2 mixed); incidence was 40.2 infections/100 person-years (95% CI, 37.9–42.7). Recurrent P vivax infections predominated onwards from 2013. According to PCR data, submicroscopic predominated over microscopic infections, especially in periods of low transmission. The integration of parasitological, entomological, and environmental observations evidenced an intense and seasonal transmission resilient to standard control measures in Lupuna and a persistent residual transmission after severe outbreaks were intensively handled in Cahuide.
Conclusions
In 2 exemplars of complex local malaria transmission, standard control strategies failed to eliminate submicroscopic and hypnozoite reservoirs, enabling persistent transmission.
Keywords: Amazon, human biting rate, Malaria, Peru, transmission
This intensive 3-year population-based cohort study in 2 contrasting settings in the Peruvian Amazon demonstrated the complexity of P falciparum and P vivax coendemicity, driven by the complex interplay of human behavior, parasite biology, and environmental determinants of mosquito prevalence.
Malaria in the Americas declined from 673 723 reported cases in 2010 to 451 242 in 2015 [1]. In Peru, for instance, the Amazonian department of Loreto (~95% of Peruvian cases) [2] quintupled the reported malaria incidence by Plasmodium vivax (12 597 to 47 671 cases) and by Plasmodium falciparum (2296 to 9208 cases) from 2010 to 2015 [3]. Leading explanations for this resurgence highlighted the lack of a long-term national malaria control plan able (1) to continue and sustain achievements attained in previous years with support of international donors [4–6] and (2) to anticipate and react to dramatic environmental changes such as severe flooding in riverine villages, like the flooding that occurred in 2012.
Designing one-size-fits-all national malaria control strategies applicable to local situations is not straightforward, given highly heterogeneous and changing malaria microepidemiologies driven by the complex local interactions among Plasmodium parasites, human behavior, and the vector habitants influenced by the environment [3]. Data obtained over the past decade in Loreto villages suggest that malaria infections missed by traditional surveillance (ie, asymptomatic and submicroscopic infections, and carriers of P vivax hypnozoites) [7–9] together with human movement related to work [10] and highly anthropophilic Nyssorhynchus darlingi mosquitoes biting frequently outdoors [11] can result in the human reservoirs of Plasmodium parasites moving malaria transmission across space and time. Knowledge gaps remain to be addressed, particularly to quantify how and to what extent silent malaria reservoirs contribute to the resilience to interventions and the sustaining of malaria transmission [12].
Large prospective population cohorts with rigorous follow-up [13, 14] aim to deepen the understanding of local malaria complexity in the Peruvian Amazon, accurately estimate the burden of silent malaria reservoirs, and explore their impact on malaria transmission in different ecological settings. This study estimates population-based incidence rates of malaria between January 2013 and December 2015 in 2 different ecological settings in the Peruvian Amazon: Lupuna (LUP) with riverine environment and Cahuide (CAH) with road-associated deforestation. These data, combined with entomological and genetic parasite diversity data, enable better understanding of temporal and spatial dynamics of malaria transmission.
METHODS
Study Area
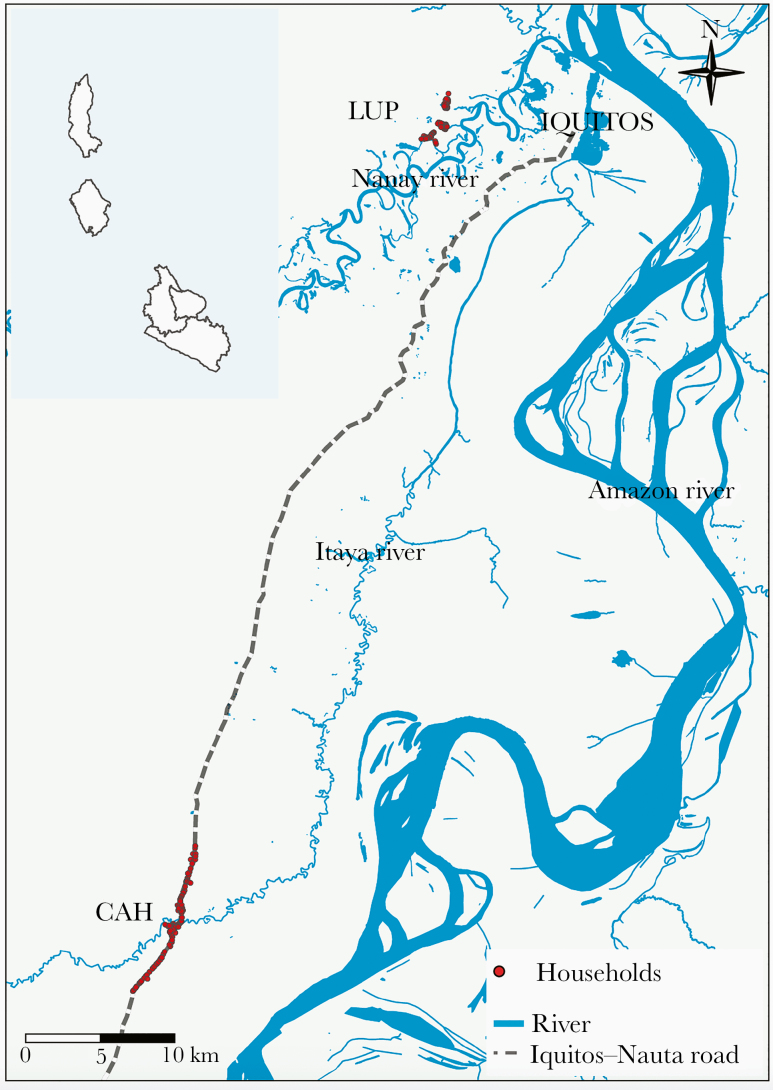
The study sites LUP and CAH (Figure 1) have been previously described. December–May and June–November are typically the tropical rainy and dry periods in the area [8]. Between 2011 and 2015, however, unusually heavy rains generated earlier and higher river level peaks (compared with historical averages), exceeding the threshold for imminent flooding for several weeks (especially in 2012 and 2015) [16].
Figure 1.
Study area. Adapted from Figure 1 in [15]. Abbreviations: LUP, Lupuna; CAH, Cahuide.
Passive case detection (PCD) data (2009–2012) indicate that malaria is seasonal (March–June) in LUP, predominantly due to P vivax [7]. Before the present study, routine malaria interventions in LUP were PCD and long-lasting insecticidal nets (LLINs) delivered in 2008 and 2010 [17]. During the study period, indoor residual spraying (IRS) with 5% deltamethrin was conducted in August 2012, April 2013, October 2013, and December 2014 [11, 18]. Malaria in CAH occurred at low levels from 2009 to 2011 [7]. After May 2012, 2 successive severe malaria outbreaks occurred, triggering 6 rounds of population screening-treatment interventions from May to December 2012 including IRS in May–June 2012 and distribution of 1 LLIN per household in July 2012 [7]. Indoor residual spraying was also conducted in March 2013 and November 2014 [18].
Field Procedures
A 3-year, population-based, longitudinal cohort study was conducted from January 1, 2013 to December 31, 2015, after census July–August 2012, baseline parasitological survey (microscopy, quantitative polymerase chain reaction [qPCR]) September–October 2012 [7], and enrollment November–December 2012. The Ethical Committee of Universidad Peruana Cayetano Heredia (SIDISI code no. 57395) and UCSD Human Subjects Protection Program (Project no. 100765) approved the study protocol.
Residents ≥3 years old providing written informed assent/consent were enrolled. Sample size estimation assumed the following: 20% residents had at least 1 microscopically confirmed malaria infection annually; 2% precision; 25% loss to follow-up; 80% power; and 5% significance level.
Cohort follow-up combined routine PCD at health posts (6 days/week), weekly active case detection of symptomatic individuals (wACDS), and monthly population screenings (mPS). Passive case detection relied on care-seeking behavior of individuals with malaria-compatible symptoms at health posts, where axillary temperature was taken and microscopy-directed treatment was done as appropriate. Household visits enabled weekly registration of axillary temperature and any malaria-compatible symptom. The mPS, conducted in the first visit of each calendar month, involved finger-prick blood sampling for microscopy [19] and dried blood spots (Supplementary Text 1). The wACDS, in the remaining visits (2th–5th) of each month, collected blood samples for microscopy from participants who had malaria-compatible symptoms within the past 7 days. Microscopically confirmed infections in the baseline survey, PCD, wACDS, and mPS were treated by a health worker according to national guidelines [20]. Patients with P vivax infections were given chloroquine for 3 days (10 mg/g on days 1 and 2, and 5 mg/kg on day 3) plus primaquine for 7 days (0.5 mg/kg per day), whereas patients with P falciparum infections were given mefloquine (12.5 mg/kg per day for 2 days) plus artesunate (4 mg/kg per day for 3 days). Although only the first day of treatment was directly observed, trained community health workers in sites and weekly visits of our field research teams enhanced treatment adherence. During weekly visits, >95% of participants with a positive microscopy in the previous 2 weeks reported that they had taken the prescribed antimalarial drugs.
DATA ANALYSIS
Cohort and Incidence Data
Every individual with positive microscopy during the cohort follow-up was defined as having a malaria infection, as long as he/she had not had prior infection with the same species in the previous 14 days. This definition prevented double counting of infections. The additional presence or history of fever, headache, chills, or general discomfort in the previous 7 days defined symptomatic malaria.
Incidence rates were calculated as the number of incident infections (×100) divided by the total person-months at risk in a given period from 2013 to 2015. A person-month indicated at least 1 follow-up by any method during the month, and the person-years (PY) were the cumulative person-months divided by 12. It is noteworthy that incidence in years 2012 and 2016 were estimated using weekly reported malaria data from national surveillance [21].
Differences in incidence by age, gender, and residency time were assessed using trend charts, 95% Byar’s confidence intervals (95% CIs), and multivariate mixed-effects negative binomial models in R v.3.6.1. Final models yielded an adjusted incident rate ratio (Adj. IRR), indicating a change in the risk from an unexposed to an exposed group. Poisson spatial scan statistics identified clusters of households with high incidence (Supplementary Text 1) [22].
Malaria Prevalence, Entomological, and River Level or Hydrological Data
Malaria prevalence by qPCR [23] and the proportion of submicroscopic infections (qPCR + microscopy−) were estimated quarterly from March 2013 to September 2015 (mPS data) and compared among subgroups with χ 2 tests and post hoc tests (Bonferroni correction). Entomological data from 12-hour mosquito collections by human landing catch before the study in 2012, from January to June in 2013–2015, and in August, October, and December 2013–2014 [11, 18] were used to estimate monthly human biting rates (HBRs). Daily water levels of the Amazon River were averaged monthly [24]. The relationships of monthly incidence rates to HBRs, river levels, and malaria prevalence were assessed with trend charts and correlation analysis.
RESULTS
Cohort Characteristics
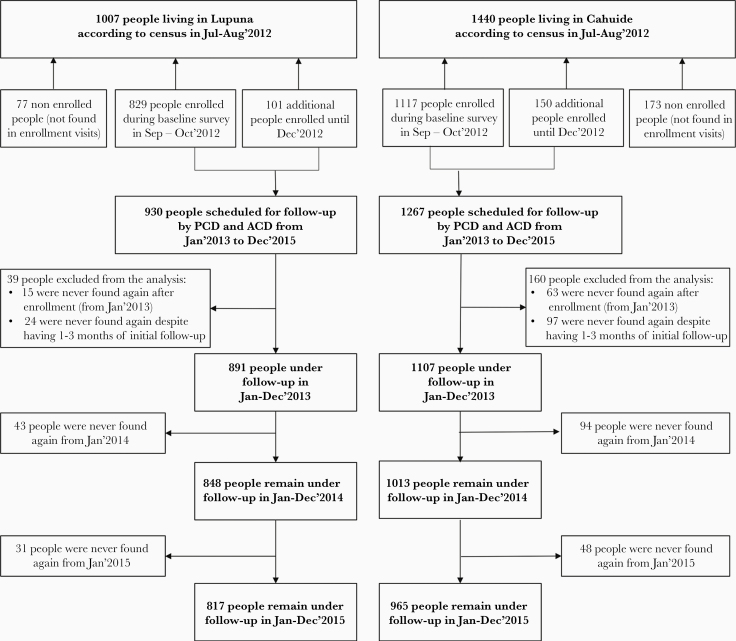
A total of 1988 participants (LUP, 891; CAH, 1107) were analyzed in the cohort from 2447 censused people (Figure 2; Supplementary Table 1). Unlike CAH, LUP was primarily inhabited by long-term residents (P < .001). More participants reported malaria during their lifetime in LUP (73.5%) than in CAH (64.0%) (P < .001) at enrollment, but malaria in the past 12 months was 3 times more common in CAH than in LUP (P < .001) (Supplementary Table 2).
Figure 2.
Flow chart of the cohort participants.
Incidence of Microscopically Confirmed Malaria Infections
Participants were followed-up between 4 and 36 months in LUP (median, 34; interquartile range [IQR], 28–36) and CAH (median, 32; IQR, 25–35). In LUP, 1708 P vivax infections were detected by microscopy in 627 participants (227 with single infections, 400 with 2–10 recurrent infections), and 120 P falciparum infections were detected in 113 participants (106 with single infections, 7 with 2 infections); determining average incidence rates of 80.7 infections/100 PY (95% CI, 77.1–84.5) (Table 1). Approximately 40% of these infections occurred in 2014 (97.1 infections/100 PY, IRR = 1.3, 95% CI = 1.2–1.5, compared with 2013).
Table 1.
Incidence Rates of Microscopically Confirmed Malaria Infections by Study Site
| Lupuna (2264.2 PY) | Cahuide (2598.9 PY) | |||||
|---|---|---|---|---|---|---|
| Type of malaria infection | Infections | Rate (/100 PY) | 95% CI | Infections | Rate (/100 PY) | 95% CI |
| Plasmodium vivax a | 1708 | 75.4 | 71.9–79.1 | 1024 | 39.4 | 37.0–41.9 |
| Plasmodium falciparum a | 120 | 5.3 | 4.4–6.3 | 20 | 0.8 | 0.5–1.2 |
| Mixed infection | 0 | 0.0 | 0 | 2 | 0.1 | 0.0–0.2 |
| Overalla | 1828 | 80.7 | 77.1–84.5 | 1046 | 40.2 | 37.9–42.7 |
| Asymptomatic P vivaxa | 667 | 29.5 | 27.3–31.8 | 601 | 23.1 | 21.3–25.0 |
| Symptomatic P vivaxa | 1041 | 46.0 | 43.2–48.8 | 423 | 16.3 | 14.8–17.9 |
| Asymptomatic P falciparuma | 41 | 1.8 | 1.3–2.4 | 11 | 0.4 | 0.2–0.7 |
| Symptomatic P falciparuma | 79 | 3.5 | 2.8–4.3 | 9 | 0.3 | 0.2–0.6 |
Abbreviations: CI, confidence interval; PY, person-years.
a P < .05.
In CAH, 1024 P vivax infections were detected in 569 participants (314 with single infections, 255 with 2 to 9 recurrent infections), 20 P falciparum infections in 19 participants (18 with single infections, 1 with 2 infections), and 2 mixed infections; yielding incidence rates of 40.2 infections/100 PY (95% CI, 37.9–42.7) (Table 1). The vast majority of infections (68.1%) occurred in 2013 (74.9 infections/100 PY, IRR = 4.6, 95% CI = 3.8–5.6, compared with 2014).
The proportion of participants with any P vivax infection detected by microscopy was similar between sites during the first year (LUP, 41.5%; CAH, 40.8%; P > .05), but it differed at the end of the study (LUP, 70.4%; CAH, 51.4%; P < .001). Asymptomatic P vivax infections barely exceeded symptomatic ones in LUP, with the lowest (~0.3) and highest (~0.55) proportions (among total P vivax infections) in the first months of 2013 and 2015, respectively (Supplementary Figure 1). In CAH, the proportion of asymptomatic P vivax infections negatively correlated with P vivax incidence (Spearman’s rho [rs] = −0.59, P < .001), presenting the highest levels (0.7–1.0) in months with the lowest incidence in 2014 and 2015. The proportion of asymptomatic P falciparum infections varied widely, mainly due to the low number of infections (Supplementary Figure 2).
Factors Associated With High Malaria Incidence
Quarterly and yearly incidence rates stratified by demographic groups consistently showed increased P vivax incidence in children aged 8–14 years over the study period in LUP (Figure 3A, Supplementary Figures 3A–4A, Supplementary Table 3). Interaction between age and time of residency in the multivariate model indicated that the association between high P vivax incidence and children was significant only among long-term residents (Adj. IRR for age3-7y = 2.9, 95% CI = 2.1–3.8; Adj. IRR for age8-14y = 3.2, 95% CI = 2.7–4.1; compared with age >44 years) (Table 2; Supplementary Figures 5A-6A). Increased P vivax incidence was associated with short-term residency among individuals aged 15–44 years (Adj. IRR = 2.3, 95% CI = 1.7–3.0) and those >44 years (Adj. IRR = 2.7, 95% CI = 1.7–4.7). It is interesting to note that interyear variations in P vivax incidence rates were wider in long-term residents aged 3–7 years (IRR2014-2013 = 0.6, 95% CI = 0.4–0.8; IRR2014-2015 = 0.7, 95% CI = 0.5–1.0) compared with those aged 8–14 years (IRR2014-2013 = 0.7, 95% CI = 0.4–0.8; IRR2014-2015 = 0.8, 95% CI = 0.6–1.0) (Supplementary Tables 4 and 5).
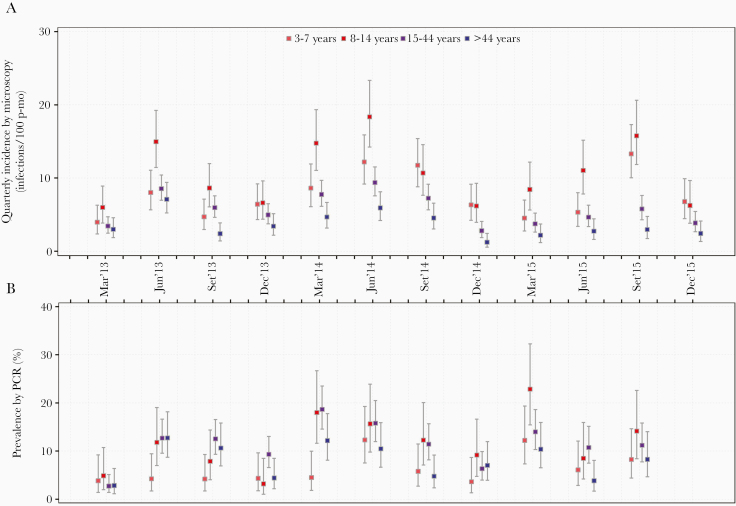
Figure 3.
Quarterly incidence of P. vivax malaria infections detected by microscopy and prevalence by PCR stratified by age groups in Lupuna from January 2013 to December 2015. Dot plots show incidence/prevalence mean rates, and 95% confidence limits (95% CI) for each age group. The Byar’s approximation and the Wilson score method were respectively used to estimate CI for incidence and prevalence rates. Incidence rates were calculated for the periods January-March, April-June, July-September and October-December of each year. Significant differences in incidence rates between age groups were found in almost all trimesters (except January-March 2013 and September-December 2013) (P < .001), showing mostly higher incidence rates in individuals aged 8-14 years in comparison with those aged 15-44 years and >45 years (pair-wise post-hoc test, P < .05). Significant differences in prevalence between age groups were found in June 2013, March 2014, and March 2015 (P < .05). Pair-wise post-hoc tests showed that children aged 3-7 years had lower prevalence than individuals aged 15-44 years (P = .04) and >44 years (P = .04) in June 2013. Children aged 3-7 years had lower prevalence than those aged 8-14 years (P = .004) and 15-44 years in March 2014 (P = .001). While in March 2015, individuals aged 8-14 years had higher prevalence than those aged >44 years (P = .008).
Table 2.
Uni- and Multivariate Risk Factor Analysis for Incidence of Microscopically Confirmed Plasmodium vivax Malaria Infections in LUP and CAH
| Infections | PY | Rate (Infections/100 PY) | IRR | Adjusted IRR | ||||
|---|---|---|---|---|---|---|---|---|
| Demographic | n | PY | Rate | 95% CI | IRR | 95% CI | IRR | 95% CI |
| Lupuna | - | - | - | - | - | - | - | - |
| Year | - | - | - | - | - | - | - | - |
| 2013 | 581 | 830.5 | 70.0 | 64.4–75.8 | Ref. | Ref. | ||
| 2014 | 679 | 751.6 | 90.3 | 83.7–97.3 | 1.3a | 1.2–1.4 | 1.3a | 1.2–1.4 |
| 2015 | 448 | 682.1 | 65.7 | 59.8–72.0 | 0.9 | 0.8–1.1 | 0.9 | 0.8–1.1 |
| Gender | - | - | - | - | - | - | - | - |
| Female | 871 | 1141.1 | 76.3 | 71.4–81.5 | Ref. | Ref. | ||
| Male | 837 | 1123.1 | 74.5 | 69.6–79.7 | 1.0 | 0.8–1.1 | 1.0 | 0.8–1.1 |
| Age | - | - | - | - | - | |||
| 3–7 y | 372 | 404.0 | 92.1 | 83.1–101.8 | 2.1a | 1.7–2.6 | ||
| 8–14 y | 436 | 341.5 | 127.7 | 116.1–140.1 | 3.0a | 2.5–3.8 | ||
| 15–44 y | 650 | 941.8 | 69.0 | 63.9–74.5 | 1.6a | 1.3–1.9 | ||
| >44 y | 250 | 576.9 | 43.3 | 38.2–49.0 | Ref. | |||
| Residency (Time) | - | - | - | - | - | - | ||
| 0–5 y | 468 | 458.9 | 102.2 | 93.1–111.5 | 1.5a | 1.3–1.8 | ||
| >5 y | 1240 | 1805.3 | 68.7 | 64.9–72.6 | Ref. | |||
| Interaction Agea Residency |
- | - | - | - | - | |||
| For Those With Residency 0–5 y | - | - | - | - | - | |||
| Age 3–7 y | - | - | - | - | - | 0.8 | 0.5–1.2 | |
| Age 8–14 y | - | - | - | - | - | 1.3 | 0.7–2.6 | |
| Age 15–44 y | - | - | - | - | - | 1.2 | 0.7–2.0 | |
| Age >44 y | - | - | - | - | - | Ref. | ||
| For Those With Residency >5 y | - | - | - | - | - | |||
| Age 3–7 y | - | - | - | - | - | 2.9a | 2.1–3.8 | |
| Age 8–14 y | - | - | - | - | - | 3.2a | 2.7–4.1 | |
| Age 15–44 y | - | - | - | - | - | 1.5a | 1.2–1.8 | |
| Age >44 y | - | - | - | - | - | Ref. | ||
| For Those Aged 3–7 y | - | - | - | - | - | |||
| Residency 0–5 y | - | - | - | - | - | 0.7b | 0.6–1.0 | |
| Residency >5 y | - | - | - | - | - | Ref. | ||
| For Those Aged 8–14 y | - | - | - | - | - | |||
| Residency 0–5 y | - | - | - | - | - | 1.1 | 0.7–1.7 | |
| Residency >5 y | - | - | - | - | - | Ref. | ||
| For Those Aged 15–44 y | - | - | - | - | - | |||
| Residency 0–5 y | - | - | - | - | - | 2.3a | 1.7–3.0 | |
| Residency >5 y | - | - | - | - | - | Ref. | ||
| For Those Aged >44 y | - | - | - | - | - | |||
| Residency 0–5 y | - | - | - | - | - | 2.7a | 1.7–4.7 | |
| Residency >5 y | - | - | - | - | - | Ref. | ||
| Cahuide | ||||||||
| Year | ||||||||
| 2013 | 695 | 948.9 | 73.2 | 67.9–78.8 | Ref. | Ref. | ||
| 2014 | 136 | 860.0 | 15.8 | 13.3–18.6 | 0.2a | 0.2–0.3 | 0.2a | 0.2–0.3 |
| 2015 | 193 | 790.0 | 24.4 | 21.2–28.1 | 0.3a | 0.3–0.4 | 0.3a | 0.3–0.4 |
| Gender | ||||||||
| Female | 472 | 1276.1 | 37.0 | 33.8–40.4 | Ref. | Ref. | ||
| Male | 552 | 1322.8 | 41.7 | 38.4–45.3 | 1.1b | 1.0–1.3 | 1.1 | 1.0–1.3 |
| Age | ||||||||
| 3–7 y | 148 | 551.6 | 26.8 | 22.8–31.4 | Ref. | Ref. | ||
| 8–14 y | 249 | 575.8 | 43.2 | 38.1–48.9 | 1.5a | 1.2–2.0 | 1.4a | 1.1–1.7 |
| 15–44 y | 418 | 966.8 | 43.2 | 39.2–47.5 | 1.6a | 1.3–2.0 | 1.4a | 1.1–1.7 |
| >44 y | 209 | 504.8 | 41.4 | 36.1–47.3 | 1.5a | 1.2–2.0 | 1.3b | 1.0–1.7 |
| Residency (Time) | ||||||||
| 0–5 y | 437 | 1321.7 | 33.1 | 30.1–36.3 | Ref. | |||
| >5y | 587 | 1277.3 | 46.0 | 42.4–49.8 | 1.4a | 1.2–1.7 | 1.3a | 1.1–1.6 |
Abbreviations: CAH, Cahuide; CI, confidence interval; IRR, incident rate ratio; LUP, Lupuna; PY, person-years; Ref., reference; y, years.
a P < .05.
b P between .1 and .05.
In CAH, stratification of incidence rates by age showed increased P vivax incidence rates at >7 years and residence times >5 years, especially during 2013 (P < .05) (Supplementary Figures 7A–9A, Supplementary Table 6). Over the cohort period, the multivariate model confirmed this increased P vivax incidence in individuals aged 8–44 years (Adj. IRR = 1.4, 95% CI 1.1–1.7, compared with age 3–7 years) and in long-term residents (Adj. IRR = 1.3, 95% CI = 1.1–1.6). Regarding P falciparum, males (Adj. IRR = 2.0, 95% CI = 1.4–2.9) and individuals >7 years (Adj. IRR ~2.5) had highest incidence rates in LUP (Supplementary Table 7).
Recurrent Infections Among Total Plasmodium vivax Infections
The proportion of recurrent infections detected by microscopy (after a prior infection) steadily increased in both sites during the first year (from 0 to 0.63 in LUP, and to 0.75 in CAH). Afterwards, smaller increases were observed in LUP (maximum = 0.92) or fluctuated between 0.56 and 0.83 in CAH (Supplementary Figure 1). Among long-term residents in LUP, individuals aged 8–14 years had the highest proportion of recurrent infections (P < .001). No significant differences were found among short-term residents in LUP and total residents in CAH (Supplementary Figure 10).
Spatial Clusters of High Malaria Incidence
Clusters of high P vivax incidence in 2013 and 2014 were consistently found in (1) southwest LUP (2013 risk ratio [RR] = 1.7, P < .001; 2014 RR =1.5, P = .02) and (2) the junction of the Iquitos-Nauta road and Itaya River in CAH (2013’s RR = 1.7, P < .001; 2014’s RR = 2.4, P < .001) (Supplementary Figures 11 and 12). In 2015, the P vivax cluster in CAH (RR = 4.3, P < .001) remained close to the junction, but it was small. There were no significant spatial clusters for P vivax in LUP in 2015, nor for P falciparum in LUP and CAH in 2013, 2014, and 2015.
Quarterly Malaria Prevalence by Quantitative Polymerase Chain Reaction
Lowest and highest malaria prevalence in LUP were observed in October 2012–March 2013 (≤5%) and March 2014 (20.5%) and December 2014 (1.7%) and June 2013 (14.4%) in CAH (Figure 4; Supplementary Tables 8 and 9). Positive correlation between prevalence by PCR and incidence rates was only found in LUP (rs vivax= 0.64, P = .03; rsfalciparum= 0.67, P = .02). Submicroscopic P vivax infections predominated over microscopic ones in both sites, with highest proportions in December 2014–March 2015 in LUP (~0.8) and in September 2014–March 2015 in CAH (>0.9). High proportions in CAH were associated with low clinical incidence rates (rs = −0.84, P = .001).
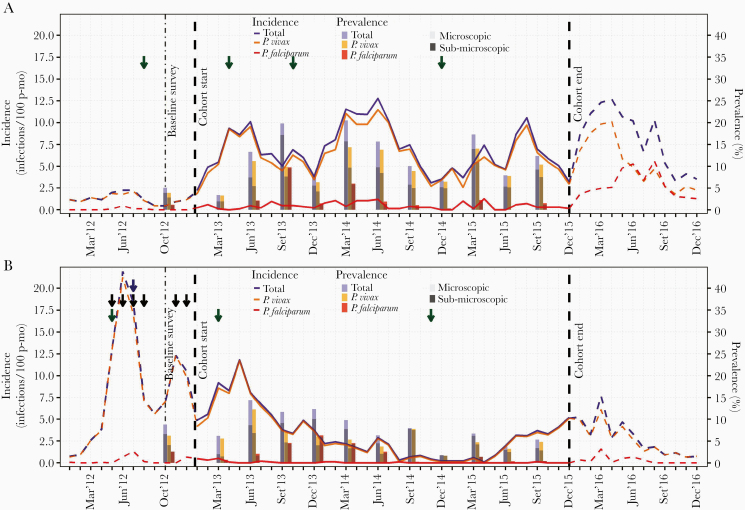
Figure 4.
Evolution of monthly incidence of malaria infections by microscopy, and quarterly prevalence by qPCR in Lupuna (A) and Cahuide (B) from January 2012 to December 2016. Dashed lines represent incidence in 2012 and 2016 estimated with surveillance data, while solid lines in 2013-2015 with cohort data. Green, blue, and black arrows indicate months when respectively indoor residual spraying (IRS) with 5% deltamethrin, distribution of long-lasting insecticidal nets (LLINs), and population screenings using microscopy and treatment of confirmed infections were conducted. The left Y-axis indicates incidence rates (number of infections/100 person-months), while the right Y-axis prevalence in percentage (%). see online version for color figure.
Stratified analysis of quarterly prevalence by demographic groups in LUP identified lowest P vivax prevalence at ages 3–7 years in June 2013 and March 2014 (P < .05) and highest prevalence at ages 8–14 years in March 2015 (P < .05) (Figure 3B; Supplementary Figures 3B–9B). In CAH, males had higher prevalence than females in September 2013 and March 2014 (P = .02) and in long-term residents in June 2014 (P = .04).
Relationship Between Entomological/Environmental Variables and the Incidence of Microscopic Malaria
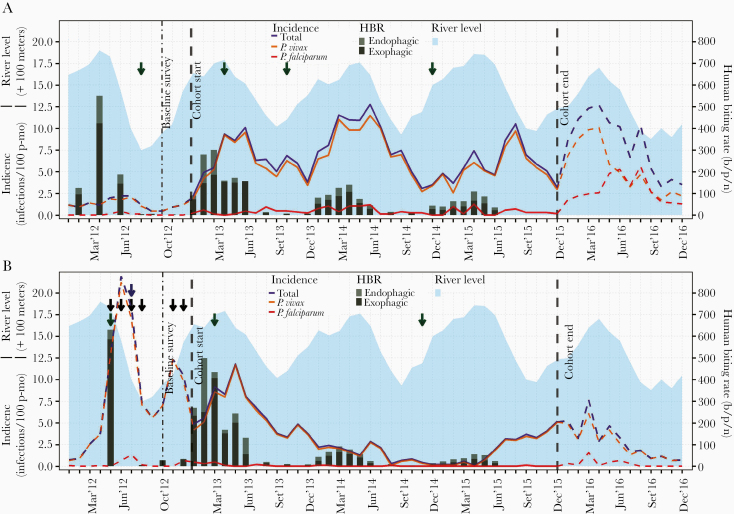
Amazon River levels from 2012 to 2015 correlated with HBRs in both sites (rs = 0.64, P < .001). Significant relationships between overall incidence rates and environmental and entomological variables during the cohort period (when found) occurred at 2-month lag times, with river levels in LUP (rs = 0.54, P = .001) and with HBRs in CAH (rs = 0.68, P < .001) (Figure 5).
Figure 5.
Evolution of monthly incidence of malaria infections by microscopy, Amazon river levels and human biting rates (HBR) in Lupuna (A) and Cahuide (B) from January 2012 to December 2016. Dashed lines represent incidence in 2012 and 2016 estimated with surveillance data, while solid lines in 2013-2015 with cohort data. HBRs were estimated using published entomological data from 12-h mosquito collections by human landing catch (HLC) before the study in 2012, from January to June in 2013-2015, and in August, October and December 2013-2014. Monthly water levels of the Amazon River were calculated with daily data from the Peruvian Amazon Service of Hydrography and Navigation. Green, blue, and black arrows indicate months when respectively indoor residual spraying (IRS) with 5% deltamethrin, distribution of long-lasting insecticidal nets (LLINs), and population screenings using microscopy and treatment of confirmed infections were conducted. The left Y-axis indicates incidence rates (number of infections/100 person-months) and river levels (meters above 100), while the right Y-axis human biting rates (bites/person/night). see online version for color figure.
Discussion
This densely sampled, prospective, longitudinal, population-based cohort study is unique among the few such studies aimed at understanding heterogeneous and changing malaria transmission dynamics in P vivax and P falciparum coendemic areas [25–27]. The assessment of parasitological rates across time and space, together with published data of genetic parasite diversity [28], entomological observations, river height measurements, and contextual surveillance/control information, provide valuable insights into contrasting epidemiological scenarios in the Peruvian Amazon. Residual transmission persisted after effectively controlling severe outbreaks in a malaria-epidemic prone site (CAH), and under conditions of intense, seasonal malaria transmission resistant to standard control measures (LUP). These sites epitomize the microheterogeneity of malaria transmission in environmentally diverse Amazonian settings, and they are generalizable to other coendemic P vivax and P falciparum settings.
The location of malaria clusters areas of flood risk [7] and the association of HBRs and malaria incidence support the hypothesis that environmental changes drove increased abundance and wide dispersal of N darlingi leading to the 2012–2013 epidemic transmission in CAH. Malaria incidence in CAH in 2012 was similar to the worst epidemic recorded in Loreto (1996–1998) [29]. In 2013, incidence decreased, but malaria transmission remained high despite outbreak responses and intense microscopy-directed treatment.
Standard malaria surveillance is inherently limited in eliminating residual infections, as demonstrated by persistent malaria prevalence in 2014 and early 2015 [30]. Human mobility, not vector movement, was most likely responsible for dispersing P vivax and P falciparum infections from outside CAH. Published changes in the genetic diversity of P vivax infections during and after malaria outbreaks in CAH are consistent with this proposed mechanism of maintenance P vivax transmission [28]. Indeed, CAH’s population is not stable, as indicated by the proportion of recent immigrants (>25% individuals with <2 years of residency) and the proportion of participants (one fourth) lost to follow-up due to migration. The accessibility to the 120-km road connecting the major city Iquitos to rural Nauta and to a riverine port make CAH a vulnerable area to imported malaria [31, 32], because of intense human movement related to work and social interactions, within the same community and in proximity to other endemic communities [7, 10]. With favorable conditions for vector development and high human mobility patterns, both endemic and imported strains would have dispersed rapidly across CAH villages and led to uncontrollable malaria outbreaks in 2012–2013 and the resurgence of malaria in all villages in late 2015 (year with floods, but less intense than in 2012). Hence human behavior converts anophelism without malaria to endemic malaria transmission.
Lupuna also withstood floods, more severe in 2012 than in 2013. The spatial cluster of highest incidence close to Nanay River and the rise of seasonal HBRs [11, 18] underlined the contribution of this environmental change to the increase in incidence rates by both P vivax and P falciparum in 2013. Integrating epidemiological and genotyping data was necessary to understand why malaria driven by floods in LUP did not lead to early increases in the malaria burden since 2012. The low prevalence of parasitemic people (mainly submicroscopic) in October 2012 and the consistent seasonal patterns of low malaria incidence (2009–2012) in the relatively stable LUP population (long residency time and high levels of study retention) suggest that asymptomatic and low-parasite density infections were able to maintain low seasonal malaria transmission before 2013 [7]. These findings also suggest that population exposure to local parasite strains was sufficient for the development of clinical immunity to malaria disease and high-density parasitemia in the precohort period [33, 34]. However, this transmission scenario would have been altered with the introduction and spread of new P vivax strains after March 2013 given microsatellite characterization of parasite populations [28]. In 2013, unlike 2012, increased human-vector contacts not only intensified the transmission of local strains, but they also facilitated the spread of new imported ones. As a result, the number of P vivax incident infections (mostly symptomatic) reached unusual peaks in high transmission season of 2013, and participants with less-developed immunity such as children and with short-time residency were the most affected groups [27, 35].
The increased contribution of recurrent P vivax infections to malaria incidence, the same location of the most likely cluster in 2013–2014, together with the decline in human-vector contacts after less severe flooding and IRS in 2014, also suggest that P vivax relapses may have played an important role in keeping high transmission levels in LUP. The rigorous study follow-up facilitated the diagnosis and treatment of microscopic infections (mainly recurrent infections), but it failed to identify the reservoir of low-density blood-stage infections (composed of new and relapsing infections) and the reservoir of hypnozoite carriers (without blood-stage parasites), which together enabled disease across transmission seasons. The decrease in malaria incidence rates in 2015 (greater in young children than in old ones among long-term residents) would likely be due to the gradual acquisition of strain-specific P vivax immunity providing some protection to LUP’s residents against malaria disease and high-density parasitemia [27, 36, 37] rather than a true reduction in transmission levels. Indeed, the submicroscopic reservoir (mainly by P vivax) with persistent high levels (≥0.7) since December 2014 highlighted the potential epidemic risk when conditions for transmission were more favorable. Unfortunately, the decrease of detection efforts after the cohort study ended was associated with an early seasonal peak of P vivax incidence and a severe and prolonged outbreak of P falciparum in 2016.
The strengths of this study include its large sample size, 3-year prospective design, rigorous parasitological follow-up, and the integration among epidemiological, vector, and environmental data for better understanding malaria transmission dynamics. The main limitations were related to the fixed cohort design [37] and to loss of participants to follow-up (at least in part due to migration) together with the entry of new residents into the study villages after the study onset (who were not additionally enrolled). These findings may have affected incidence and prevalence estimations in unknown ways; for example, by introducing new parasite strains or by the introgression of malaria-naive individuals. However, the higher number of immigrants (12.1% in LUP and 23.8% in CAH) than emigrants (<5% according to population census at end of the cohort) may in part support the validity of our estimations at the population level.
Conclusions
The data provided here are 2 exemplars of complex local malaria transmission in the Peruvian Amazon. The changing interactions across time between the coendemic and biological distinct P falciparum and P vivax parasites and human-vector contact rates determined by human mobility and environmental-driven vector behaviors produced contrasting scenarios of malaria transmission with microheterogeneity observed within the same endemic villages. Standard control strategies may have contributed (1) to a reduction of malaria after outbreaks and (2) to possibly reducing increases in morbidity during high transmission season. However, these standard interventions did not eliminate submicroscopic parasitemics nor hypnozoite reservoirs [30, 39], enabling continued low transmission in areas with variable malaria receptivity such as CAH and areas with high seasonal transmission with relatively permanent malaria receptivity such as LUP. For instance, we observed that prevalence levels of submicroscopic infections as low as 5% can represent a risk for malaria resurgence when conditions become favorable for transmission. Easy-to-use and highly sensitive molecular tests like those based on loop-mediated isothermal deoxyribonucleic acid amplification (still under evaluation) [40] and treatment of confirmed blood-stage infections with shorter courses of effective drugs like tafenoquine (TFQ) [41, 42] will definitively improve test-and-treat interventions, but carriers of liver-stage P vivax parasites will remain unidentified [12]. Innovative strategies are needed to support malaria programs aimed to move from low to zero malaria transmission. Evidence from the dynamics of malaria transmission in CAH and LUP suggests the potential of seasonal focal drug administration with artemisinin-based combinations plus TFQ to high-risk individuals—such as individuals with malaria infections within the past 1 to 2 years, multiple recurrences, and/or with high work-related mobility—for targeting and eliminating the parasite reservoir. Modeling [43] and field studies would be required to assess the effectiveness and cost-effectiveness of this strategy before implementation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all residents and local authorities from the Loreto villages of Cahuide and Lupuna for their enthusiastic participation in the study. We also thank all field workers for their dedication during the fieldwork.
Authors’ contributions. A. L.-C., J. M. V., and D. G. conceived and designed the study. M. G.-G., H. R., M. M., and R. C. supervised the fieldwork. D. G., P. M., and R. R. supervised the laboratory assays. A. R.-A., M. G.-G., and G. C.-E. contributed to the data management and the consolidation of the fieldwork and laboratory data. A. R.-A. and N. S. conducted the analysis. A. R.-A. prepared the figures and tables and wrote the first draft of the paper. J. M. V., A. L.-C., H. R., N. S., J. E. C., P. M., and D. G. contributed to the result interpretation and writing of the paper. All authors read and approved the final manuscript.
Disclaimer. The funders had no role in study design or in preparation of the manuscript.
Financial support. This work was funded by cooperative agreement U19AI089681 from the US Public Health Service, National Institutes of Health/National Institute of Allergy and Infectious Diseases, as the Amazonian International Center of Excellence in Malaria Research. A. R.-A. is a Postdoctoral Researcher of the Fonds de la Recherche Scientifique ([FNRS] Belgium).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. World Malaria Report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 2. Griffing SM, Gamboa D, Udhayakumar V. The history of 20th century malaria control in Peru. Malar J 2013; 12:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosas-Aguirre A, Gamboa D, Manrique P, et al. . Epidemiology of Plasmodium vivax malaria in Peru. Am J Trop Med Hyg 2016; 95:133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antiporta DA, Rosas-Aguirre A, Chang J, Llanos-Cuentas A, Lescano AG. Malaria eradication. Lancet 2020; 395:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soto-Calle V, Rosas-Aguirre A, Llanos-Cuentas A, et al. . Spatio-temporal analysis of malaria incidence in the Peruvian Amazon Region between 2002 and 2013. Sci Rep 2017; 7:40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flores W, Chang J, Barillas E. Rapid assessment of the performance of malaria control strategies implemented by countries in the Amazon subregion using adequacy criteria: case study. Malar J 2011; 10:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosas-Aguirre A, Guzman-Guzman M, Gamboa D, et al. . Micro-heterogeneity of malaria transmission in the Peruvian Amazon: a baseline assessment underlying a population-based cohort study. Malar J 2017; 16:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosas-Aguirre A, Speybroeck N, Llanos-Cuentas A, et al. . Hotspots of malaria transmission in the Peruvian Amazon: rapid assessment through a parasitological and serological survey. PLoS One 2015; 10:e0137458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreno-Gutierrez D, Llanos-Cuentas A, Luis Barboza J, et al. . Effectiveness of a malaria surveillance strategy based on active case detection during high transmission season in the Peruvian Amazon. Int J Environ Res Public Health 2018; 15:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrasco-Escobar G, Miranda-Alban J, Fernandez-Miñope C, et al. . High prevalence of very-low Plasmodium falciparum and Plasmodium vivax parasitaemia carriers in the Peruvian Amazon: insights into local and occupational mobility-related transmission. Malar J 2017; 16:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moreno M, Saavedra MP, Bickersmith SA, et al. . Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J 2015; 14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bassat Q, Velarde M, Mueller I, et al. . Key knowledge gaps for Plasmodium vivax control and elimination. Am J Trop Med Hyg 2016; 95:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moss WJ, Dorsey G, Mueller I, et al. . Malaria epidemiology and control within the international centers of excellence for malaria research. Am J Trop Med Hyg 2015; 93:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rao MR. Foreword: International Centers of Excellence for Malaria Research. Am J Trop Med Hyg 2015; 93:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosas-Aguirre A, Patra KP, Calderón M, et al. . Anti-MSP-10 IgG indicates recent exposure to Plasmodium vivax infection in the Peruvian Amazon. JCI Insight 2020; 5:e130769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solano-Villarreal E, Valdivia W, Pearcy M, et al. . Malaria risk assessment and mapping using satellite imagery and boosted regression trees in the Peruvian Amazon. Sci Rep 2019; 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosas-Aguirre A, Guzmán-Guzmán M, Moreno-Gutierrez D, Rodriguez-Ferrucci H, Vargas-Pacherrez D, Acuña-González Y. [Long-lasting insecticide - treated bednet ownership, retention and usage one year after their distribution in Loreto, Peru]. Rev Peru Med Exp Salud Publica 2011; 28:228–36. [DOI] [PubMed] [Google Scholar]
- 18. Prussing C, Moreno M, Saavedra MP, et al. . Decreasing proportion of Anopheles darlingi biting outdoors between long-lasting insecticidal net distributions in peri-Iquitos, Amazonian Peru. Malar J 2018; 17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ministerio de Salud del Perú. [Norma técnica de salud para el control de calidad del diagnóstico microscópico de malaria]. Lima: MINSA; 2010. [Google Scholar]
- 20. Ministerio de Salud del Perú. [Norma técnica para la atención de la malaria y malaria severa en el Perú. NTS Nro. 054-MINSA/DGSP-V.01, modificada en Febrero 2015]. Lima, Peru: Peruvian Ministry of Health; 2015. [Google Scholar]
- 21. Ministerio de Salud del Perú. [Tendencia y situación de las enfermedades sujetas a vigilancia epidemiológica: malaria]. Bol Epidemiol 2015; 24:975– 986. [Google Scholar]
- 22. Kulldorff M SaTScan -Software for the spatial, temporal, and space-time scan statistics. Boston: Harvard Medical School and Harvard PilgrimHealth Care; 2010. [Google Scholar]
- 23. Mangold KA, Manson RU, Koay ES, et al. . Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol 2005; 43:2435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Servicio de Hidrografia y Navegación de la Amazonía. [Avisos a los navegantes fluviales]. Available at: https://www.dhn.mil.pe/shnaNEW/boletines/Avilona/Avisos/12-12-2016.pdf. Accessed 12 September 2020. [Google Scholar]
- 25. Branch O, Casapia WM, Gamboa DV, et al. . Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J 2005; 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vitor-Silva S, Siqueira AM, de Souza Sampaio V, et al. . Declining malaria transmission in rural Amazon: changing epidemiology and challenges to achieve elimination. Malar J 2016; 15:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. da Silva-Nunes M, Codeço CT, Malafronte RS, et al. . Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg 2008; 79:624– 35. [PubMed] [Google Scholar]
- 28. Manrique P, Miranda-Alban J, Alarcon-Baldeon J, et al. . Microsatellite analysis reveals connectivity among geographically distant transmission zones of Plasmodium vivax in the Peruvian Amazon: a critical barrier to regional malaria elimination. PLoS Negl Trop Dis 2019; 13:e0007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roper MH, Torres RS, Goicochea CG, et al. . The epidemiology of malaria in an epidemic area of the Peruvian Amazon. Am J Trop Med Hyg 2000; 62:247–56. [DOI] [PubMed] [Google Scholar]
- 30. Moonen B, Cohen JM, Snow RW, et al. . Operational strategies to achieve and maintain malaria elimination. Lancet 2010; 376:1592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rovira-Vallbona E, Contreras-Mancilla JJ, Ramirez R, et al. . Predominance of asymptomatic and sub-microscopic infections characterizes the Plasmodium gametocyte reservoir in the Peruvian Amazon. PLoS Negl Trop Dis 2017; 11:e0005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delgado-Ratto C, Gamboa D, Soto-Calle VE, et al. . Population genetics of Plasmodium vivax in the Peruvian Amazon. PLoS Negl Trop Dis 2016; 10:e0004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Longley RJ, Sattabongkot J, Mueller I. Insights into the naturally acquired immune response to Plasmodium vivax malaria. Parasitology 2016; 143:154–70. [DOI] [PubMed] [Google Scholar]
- 34. da Silva-Nunes M, Moreno M, Conn JE, et al. . Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally-driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop 2012; 121:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueller I, Galinski MR, Tsuboi T, Arevalo-Herrera M, Collins WE, King CL. Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv Parasitol 2013; 81:77–131. [DOI] [PubMed] [Google Scholar]
- 36. Lin E, Kiniboro B, Gray L, et al. . Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS One 2010; 5:e9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michon P, Cole-Tobian JL, Dabod E, et al. . The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 2007; 76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 38. Gal R, Monninkhof EM, van Gils CH, et al. . The Trials within cohorts design faced methodological advantages and disadvantages in the exercise oncology setting. J Clin Epidemiol. 2019; 113:137–146. [DOI] [PubMed] [Google Scholar]
- 39. Wells TNC, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010; 26:145–151. [DOI] [PubMed] [Google Scholar]
- 40. Serra-Casas E, Manrique P, Ding XC, et al. . Loop-mediated isothermal DNA amplification for asymptomatic malaria detection in challenging field settings: technical performance and pilot implementation in the Peruvian Amazon. PLoS One 2017; 12:e0185742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Llanos-Cuentas A, Lacerda MVG, Hien TT, et al. . Tafenoquine versus primaquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med 2019; 380:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lacerda MVG, Llanos-Cuentas A, Krudsood S, et al. . Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med 2019; 380:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosas-Aguirre A, Erhart A, Llanos-Cuentas A, et al. . Modelling the potential of focal screening and treatment as elimination strategy for Plasmodium falciparum malaria in the Peruvian Amazon Region. Parasit Vectors 2015; 8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.