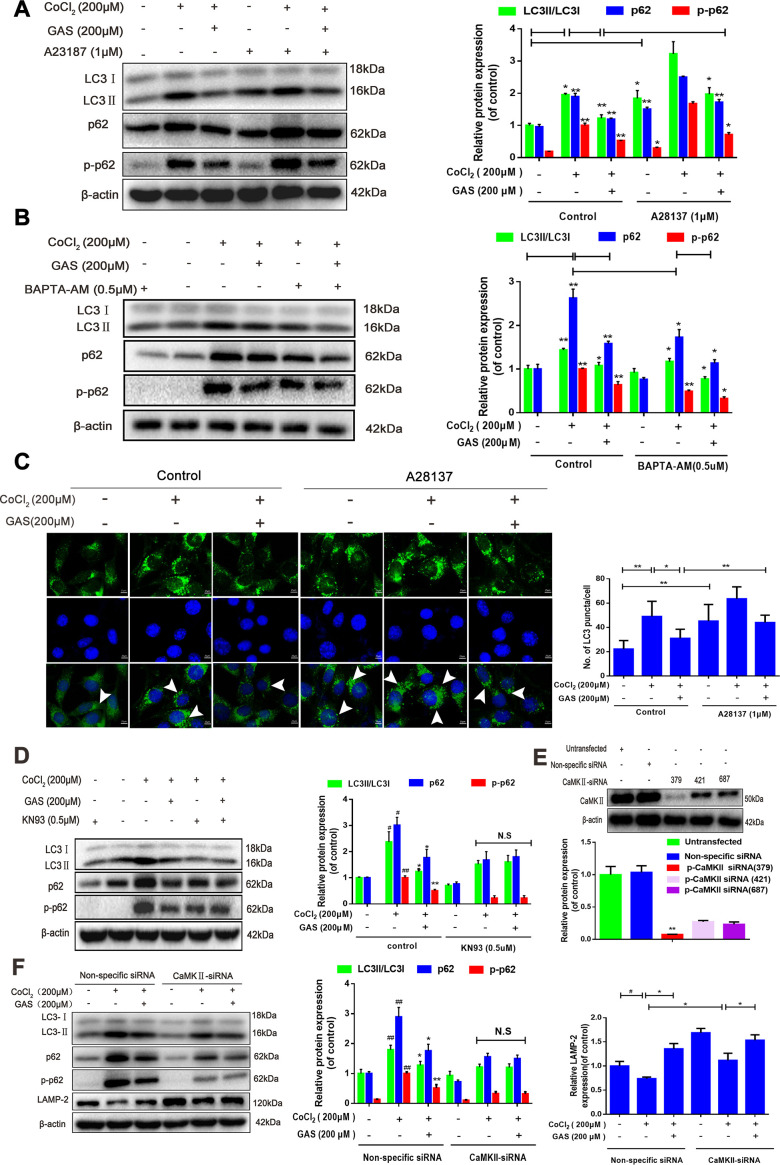
Figure 6.
GAS alleviated the CoCl2-induced suppression of autophagic flux by inhibiting [Ca2+]i-dependent CaMKII phosphorylation in HT22 cells. (A, B) Levels of LC3, p62, and phosphorylated p62 (Ser349) in HT22 cells treated with calcium chelator (BAPT-AM) or calcium ionophore (A23187) were assessed with or without GAS treatment (200 μM) for 24 h (n = 3). (C) Immunofluorescence analysis revealing modulation of LC3 in HT22 cells treated with calcium ionophore (A23187), with or without GAS (200 μM) for 24 h (n = 3). Autophagosomes were visualized (green puncta) by using a Leica DMIRB at 800× magnification. In each independent experiment, 5 visual field cells were randomly selected and quantified and expressed as mean ± standard error of the mean (SEM). (D) Levels of LC3, LAMP-2, p62, and phosphorylated p62 (Ser349) in HT22 cells treated with KN93 were assessed with or without GAS treatment (200 μM) for 24 h (n = 3). (E) Detection of CAMKII-siRNA transfection efficiency by western blotting. (F) Levels of LC3, LAMP2, p62, and phosphorylated p62 (Ser349) in HT22 cells transfected with nonspecific siRNA or CaMK II-siRNA were evaluated with or without GAS treatment (200 μM) for 24 h (n = 3). The experimental results were normalized to β-actin levels and are shown as fold changes relative to control cells.