Figure 1.
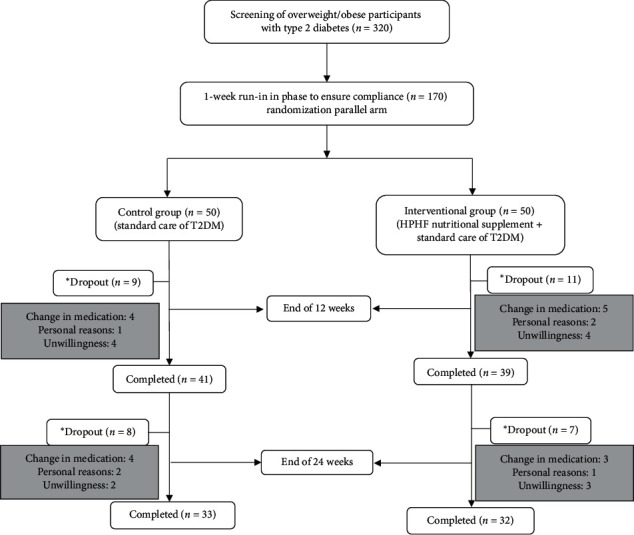
Study design. Note: a one-week run-in phase was performed before randomization to ensure compliance of participants enrolled in the study. Lipid profile was carried out at baseline (first 15 days), interim visit (15 days at the end of 12 weeks), and end of the study (15 days at the end of 24 weeks). For dietary compliance assessments, average of 5 recalls were collected during the 24 weeks' intervention and compared to baseline (3 days of dietary recall at baseline). ∗Total dropouts (n = 35): reasons include lack of response or unwillingness to continue the study, personal reasons (such as out-of-station travel and family function), and change in diabetes medication.
