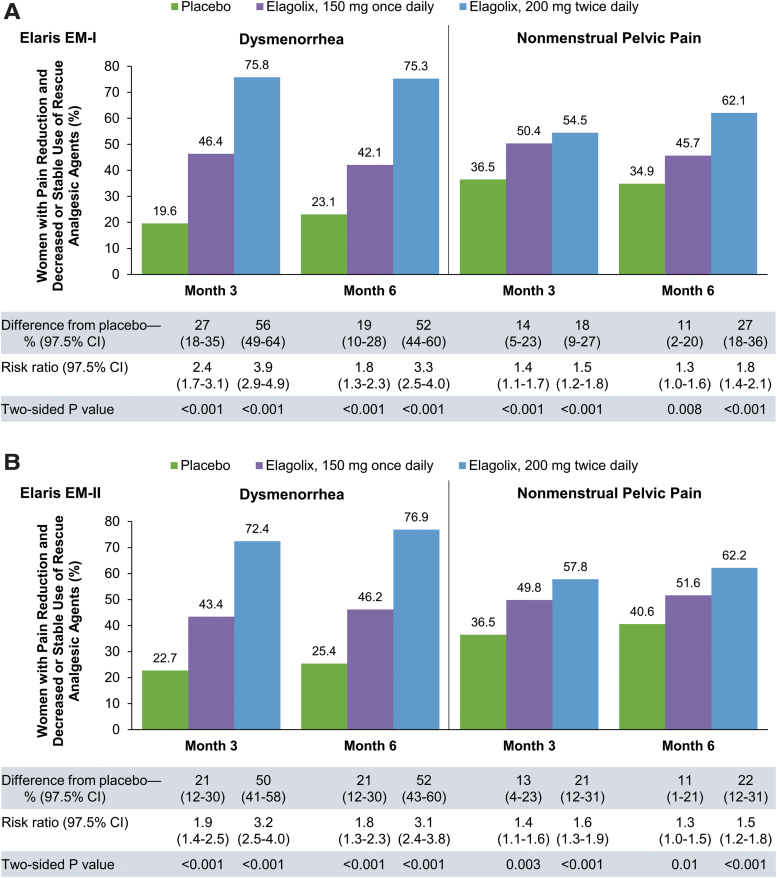
FIG. 1.
Response rates among women with moderate-to-severe endometriosis-associated pain in two phase 3, placebo-controlled clinical trials of elagolix. Primary endpoint results from the Elaris EM-I (A) and Elaris EM-II (B) clinical trials. Clinical response was defined as a clinically meaningful reduction in pain score and decreased or stable use of rescue analgesic agents. Thresholds for a clinically meaningful change from baseline were −0.81 for dysmenorrhea and −0.36 for NMPP in Elaris EM-I and −0.85 for dysmenorrhea and −0.43 for NMPP in Elaris EM-II. p-Values are for the comparison of each elagolix treatment group versus placebo. Reproduced with permission from Taylor et al.26 CI, confidence interval.