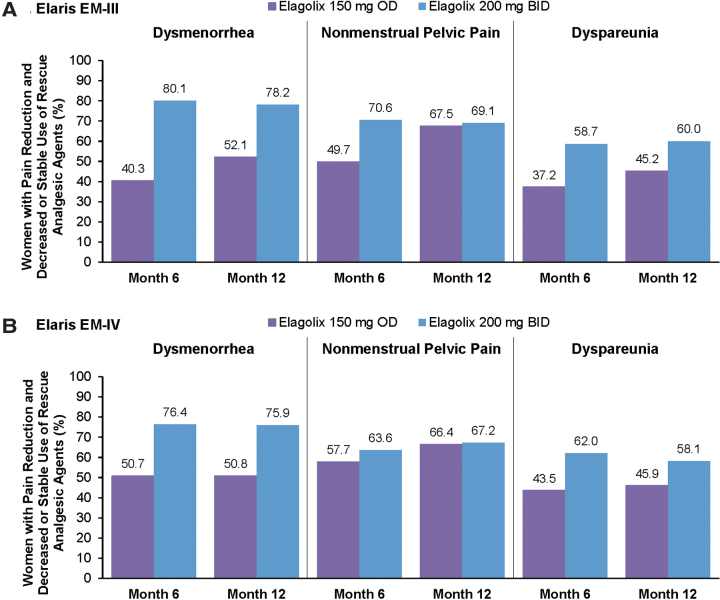
FIG. 2.
Response rates in two phase 3, long-term extension studies of elagolix for moderate-to-severe endometriosis-associated pain. Proportion of dysmenorrhea, NMPP, and dyspareunia responders in the Elaris EM-III (A) and Elaris EM-IV (B) clinical trials. Clinical response was defined as patients who experienced a clinically meaningful reduction in the respective type of pain (using the same thresholds as determined in the placebo-controlled trials)26 and decreased or stable rescue analgesic use. Data from Surrey et al.27 BID, twice daily; OD, once daily.