Figure 1.
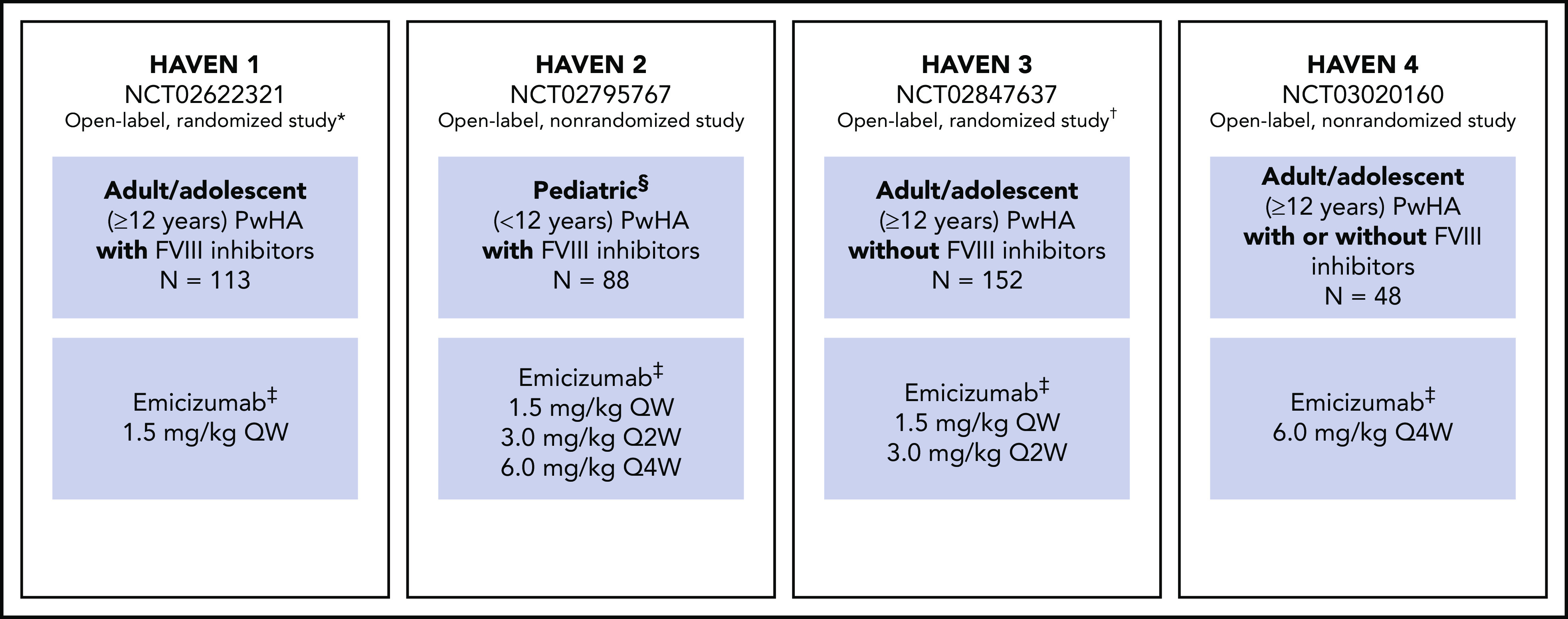
Study overview for HAVEN 1, HAVEN 2, HAVEN 3, and HAVEN 4.8-11 *Participants receiving episodic bypassing agents prior to study entry were randomized to emicizumab prophylaxis (arm A) or no emicizumab (arm B, control), and those receiving prophylactic bypassing agents prior to study entry received emicizumab prophylaxis (arm C). After completing the first 24 weeks of the trial, participants in the control arm (arm B) could receive emicizumab prophylaxis. A fourth arm also receiving emicizumab prophylaxis (arm D) consisted of participants enrolled after arms A through C closed. †Participants receiving episodic FVIII prior to study entry were randomized (2:2:1) to emicizumab once weekly (arm A), emicizumab every 2 weeks (arm B) or no prophylaxis (arm C, control), and those receiving prophylactic FVIII prior to study entry received emicizumab once weekly (arm D). ‡Maintenance doses. With the exception of the HAVEN 4 PK run-in cohort (n = 7), all maintenance doses were preceded by loading doses of 3.0 mg/kg once weekly for 4 weeks. §Adolescents aged 12 to 17 years were also eligible to enroll in HAVEN 2 if they weighed <40 kg; 3 participants were aged 12 to 17 years. QW, once weekly; Q2W, once every 2 weeks; Q4W, once every 4 weeks.
