Abstract
We determined whether the dietary supplementation with a commercial product (vegetable biocholine - VB) for Lacaune ewes at peak lactation would improve animal health and production as well as milk quality. We also determined the effects of VB as a feed additive. We used thirty Lacaune ewes at 30 days of lactation, allocated into three groups: T0 (control, without VB), T5 (5 g of VB/animal/day) and T10 (10 g of VB/animal/day). T10 sheep had greater milk yield during the experimental period. T10 ewes had also a significantly lower feed conversion ratio than the other groups. Total solids concentration of milk was higher in T10 than in the other groups on day 20. The somatic cell count in milk was lower in ewes that consumed VB in the highest dose (T10) than in the T0 and T5 in days 15 and 20. Lower levels of lipoperoxidation (LPO) and of reactive oxygen species (ROS) were observed in milk from ewes fed with the VB supplemented diet on day 20, associated with increased levels of total antioxidant capacity and superoxide dismutase activity in milk. In serum, we found that T5 and T10 animals had significantly higher levels of non-enzymatic antioxidants (non-protein thiols) associated with reduced LPO and ROS content. Higher levels of globulins were observed in T10 than in T0 sheep on day 20. These data suggest that VB dietary supplementation stimulates antioxidant responses and increases the concentration of globulins in a manner beneficial to sheep health. Milk production, feed conversion rate, and milk SCC were also improved.
Keywords: Antioxidant, Production, Lactation, Health animal, Agricultural biotechnology
Antioxidant; Production; Lactation; Health animal; Agricultural biotechnology.
1. Introduction
The demand for sheep milk is increasing in the market due to its nutritional superiority compared to cow's milk, i.e., higher total solids content (Pavic et al., 2002), despite being produced in smaller quantities (IBGE, 2018). Currently, several industries are seeking to increase production and improve the quality of animal products, as well as to achieve sustainability and maximize animal welfare in order to meet an increasingly demanding consumer market. Sheep milk has high protein and fat levels, which is desirable for the dairy industry. As a result, the production yield of cheese and yogurt increases (FAO, 2013). However, the composition of sheep's milk depends on several factors, with emphasis on nutrition (Ticiani et al., 2013). The nutritional requirements of dairy ewes increase substantially from late gestation to milk peak production. In particular, during the peripartum period, there are lower levels of endogenous antioxidants (Bernabucci et al., 2005). This condition often stresses the capacity of the liver, an organ responsible for functions such as detoxification (Adler, 1970; Baird, 1977; Rukkwamsuk et al., 1999 ). The liver is the major organ within which methylation reactions occur. Many hepatic genes are involved in pathways involved in the development of fatty liver (Mehedint and Zeisel, 2013; Walker, 2017). According to the literature, dietary intake of methyl donors such as choline influences the methylation of DNA and histones, thereby altering the epigenetic regulation of gene expression (Mehedint and Zeisel, 2013).
Choline is an essential nutrient for animals because it has lipotropic effects; it participates in processes fundamental to metabolism, including the construction and maintenance of cell structure and formation of acetylcholine (Berchielli et al., 2011). In mammals, the only endogenous pathway for choline biosynthesis is the methylation of phosphatidylethanolamine to phosphatidylcholine (PC) by phosphatidylethanolamine N-methyltransferase coupled to PC degradation (Li et al., 2005). The primary form of choline on the market is synthetic choline chloride. A new formulation, known as vegetable biocholine (VB), is being promoted because it contains phosphatidylcholine; this is important because phosphatidylcholine is transported between membranes within the cell by phosphatidylcholine transfer protein (Wirtz, 1991). Phosphatidylcholine biosynthesis is required for normal very low-density lipoprotein secretion from hepatocytes (Li and Vance, 2008), resulting in hepatoprotective and antioxidants effect as described by Souza et al. (2020) in fish supplemented with Biocholine Powder® and challenged with aflatoxin. Furthermore, an improvement in hepatic metabolism of ewes and rams was observed that was reflected in higher serum levels of non-esterified fatty acids, glucose and cholesterol available to meet animal demands (Rodriguez-Guerrero et al., 2018).
Supplementation with VB has shown potential to improve livestock production. A study reported increased weight gain in Nile tilapia of approximately 25% (Baldissera et al. (2019). Researchers demonstrated increased average daily weight gain in beef cattle by approximately 18% (Fernandes et al., 2008) and increased colostrum production in pre- and postpartum dairy cows (Valencia Narváez 2019). Recently, our research group tested the dose of 5 g/animal/day of VB for dairy sheep in the transition period. There was a tendency of reducing SCC and increasing milk production (Alba et al., 2020). These results were taken into consideration for the experimental design of the present study, in which a higher dose was used in sheep, in addition to using sheep with similar milk production at the beginning of the experiment.
We hypothesized that VB supplementation would modulate liver metabolism and would stimulate antioxidant responses. The aim of this study was to determine whether dietary supplementation with VB in Lacaune ewes at peak lactation would improve animal health and production as well as milk quality.
2. Material and methods
The experimental procedures on animals were approved by an institutional ethics committee (CEUA/UDESC), and accorded with the rules issued by the National Council for Control of Animal Experimentation (CONCEA): Number protocol 6601130319.
2.1. Animals and experimental design
The experiment was conducted at a commercial dairy sheep farm in Chapecó, Santa Catarina, Brazil. Thirty multiparous lactating sheep [30 ± 3 days (d) postpartum] of the Lacaune breed were selected according to body weight (65.6 ± 3.5 kg), age, date of lambing, and milk production and were assigned randomly to one of three treatments (ten sheep/treatment): no VB supplementation (control group; T0); 5 g VB/animal/day (T5) or 10 g VB/animal/day (T10) added in the concentrate (grain and mineral mixture).
We used the feed available on the farm in proportions determined by the owners; we only added the VB to the concentrate. The commercial product was first homogenized for 5 min with 2 kg ground corn using an electric Y mixer with a capacity of 5 kg. Then, the homogenate was added to the rest of the concentrate ingredients (horizontal mixer with a capacity of 50 kg for 5 min), to produce a homogeneous mixture.
The sheep were housed in a covered feedlot with wood shavings on the floor and were allocated to three pens (one treatment/pen) located side-by-side. The trial occurred over 20 days with first 15 days set as the adaptation period to the experimental diet with VB in concentrate. The experimental design and duration of the experiment was based on other studies (Jaguezeski et al., 2018; Alba et al., 2019a; Santos et al., 2019; Cunha et al., 2020).
The experiment was carried out in the south of Brazil, in a shed without air conditioning, with lateral openings. The experiment took place during the summer months and the temperature was measured inside the building during the day. The minimum and maximum temperatures recorded were 14.4 °C and 38.2 °C, respectively. The maximum and minimum temperatures, as well as the temperature-humidity index (THI; Mader et al., 2006) during the experimental period are presented in Supplementary Figure - Temperature, humidity and THI.
In the collective stalls, the sheep were trapped in a headlock in their feeders right after milking. Each animal received 1.2 kg/d of concentrate, approximately 4.0 kg/d of corn silage (green matter) divided into two daily feedings (0700 h and 1700 h; Table 1). Concentrate was offered first, and approximately 15 min later, silage was offered. The sheep were trapped for silage intake for approximately 1 h. However, for the purpose of determining silage intake, intake was determined per group, because after headlock the sheep were set free in the stall, with free access to the drinking fountain and feeders (leftover silage).
Table 1.
Ingredients and chemical composition of ingredients and experimental diets.
| Feeds (kg) | As fed (kg/sheep/day) | Dry matter (DM; kg/sheep/day) |
|---|---|---|
| Corn silage | 4.00 | 1.31 |
| Concentrate | 1.20 | 0.96 |
| Ingredients (g/kg of natural matter) | Concentrates |
||
|---|---|---|---|
| T0 | T5 | T10 | |
| Ground corn | 672 | 672 | 672 |
| Soybean meal | 277 | 277 | 277 |
| Calcitic limestone | 10.0 | 10.0 | 10.0 |
| Sodium bicarbonate | 4.0 | 4.0 | 4.0 |
| Premix1 |
37.0 |
37.0 |
37.0 |
|
Additive in concentrate (g/kg of natural matter) | |||
| Vegetable biocholine2 | 0.00 | 4.16 | 8.33 |
| Chemical composition3 | Corn silage | BP | Concentrates1 |
||
|---|---|---|---|---|---|
| T0 | T5 | T10 | |||
| DM, g/kg | 329 | 964 | 879 | 883 | 885 |
| Ash, g/kg DM | 42.1 | 18.0 | 65.2 | 69.3 | 67.7 |
| CP, g/kg DM | 80.1 | 47.0 | 156 | 148 | 152 |
| NDF, g/kg DM | 331 | 445 | 106 | 119 | 100 |
| ADF, g/kg DM | 179 | 384 | 45.0 | 51.0 | 46.0 |
| EE, g/kg DM | 43.9 | 57.0 | 34.3 | 36.7 | 34.7 |
| TDN, g/kg DM | 731 | 747 | 821 | 824 | 828 |
| TPC (mg GAE 100 g DM) | - | 28.2 | 9.74 | 9.85 | 9.89 |
| IC50 (μg/mL) | - | 0.30 | 5.52 | 5.37 | 5.11 |
The premix had: calcium min. 180 max. 220 g; phosphorus min. 32 g; sodium min. 40 g; sulfur min. 20 g; magnesium min. 20 g; cobalt min. 16 mg; iodine min. 17 mg; manganese min. 420 mg; selenium min. 730 mg; zinc min. 730 mg; fluorine max. 600 mg; niacin min. 500 mg; vitamin A min. 95000 IU; vitamin D min. 20000 IU; vitamin E min. 350 IU; monensin sodium 1200 mg; Saccharomyces cerevisiae 2.1 × 1010 UFC.
Vegetable choline (Biocholine Powder, Nutriquest Technofeed Company, Campinas, SP, Brazil) produced from Azadirachta indica, Citrullus colocynthis, Trachyspermum ammi, Achyranthes aspera plants was used as additive.
CP (crude protein), NDF (neutral detergent fiber), ADF (acid detergent fiber), EE (ether extract), total nutrients digestible (TDN; Calculated as described by Weiss et al., 1992), TPC (total phenolic content) and IC50 (determination of antioxidant activity by elimination of radicals by DPPH).
Every day of the experiment, 100% of the concentrate supplied to the sheep was consumed. On days 16, 17, 18, 19, and 20, intake was also measured by subtracting the weight of leftovers from the amount of feed offered daily.
2.2. Milk measurement
Individual milk production was evaluated twice a day (0600h and 1700h) at the beginning (day 0), middle (day 7) and end (day 15) of the adaptation period, and during the experimental period (days 16–20) using a True Test® meter (Auckland, New Zealand). True Test® meter is a device that is coupled to the milking system, capable of measuring milk production and collecting a homogeneous sample of the complete milking per animal.
The results of milk production were expressed as L/ewe/day. Feed conversion was expressed as DM intake/milk yield; calculated as feed intake (individual concentrate (kg/day) and average silage of the group (kg/day)) divided by milk production (L/day).
2.3. Blood and milk collection
A mixed sample (40 mL) of milk (proportional to amounts produced in the morning and evening) was collected daily per animal, using WB HI/Pullout equipment (Tru-Test®) that collects a homogeneous sample from each animal for the entire milking on days 0, 15 and 20. Two milliliters of milk sample were transferred to microtubes and stored at –20 °C for 30 days.
Blood samples were collected from the jugular vein in blood collection tubes without anticoagulant (for biochemical, oxidant and serum antioxidant analysis) at 0700h before animals were fed on days 0, 15 and 20. Immediately after collection, blood samples were stored on ice. Blood samples without heparin were centrifuged at 5100 g for 10 min. Serum was harvested and stored at –20 °C for 30 days.
2.4. Feed analysis
2.4.1. Vegetable choline
VB was purchased from Nutriquest Technofeed Company (Biocholine Powder®). The product is derived from Azadirachta indica, Citrullus colocynthis, Trachyspermum ammi, and Achyranthes aspera plants as described in the commercial product feed tag. The commercial product (Biocholine Powder®) contains guaranteed levels of total phosphatidylcholine (natural choline conjugates) of 16 g/kg of product. A sample of this additive was used for the quantification of phosphatidylcholine and phytol in VB using high-performance thin-layer chromatography, as described by Kupke and Zeugner (1978). Based on this quantification, we calculated the levels of phosphatidylcholine consumed per animal/day.
2.4.2. Chemical composition of concentrate, and silage
Feed samples were dried in a forced ventilation oven at 55 °C for 72 h, ground to 1-mm size in a Wiley mill, and analyzed according to AOAC (1990) as follows: dry matter (DM), method 967.03, ash, method 942.05, crude protein (CP), method 981.10 and EE, method 920.29. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) were analyzed using detergent solution (Van Soest, 1994) using thermostable amylase (Termamyl 120 L Novozymes A/S, Bagsvaerd, Denmark) and without sodium sulfite in a fiber extractor (Tecnal, TE-149; Tecnal, Piracicaba, SP, Brazil). NDF and ADF were not corrected for residual protein and ash. The concentration of total digestible nutrients (TDN) were calculated according to Weiss et al. (1992).
2.4.3. Determination of total phenolic content (TPC) and antioxidant activity in vegetable choline and diets
The methodologies to determination of TPC and determination of antioxidant activity by elimination of radicals by DPPH in vegetable choline and diets were written in detail by Alba et al. (2019a). The TPC and IC50 of VB and concentrates are displayed in Table 1. We found that the concentrations of TPC were low in VB, and were not different among T0, T5 and T10 diets, suggesting that VB supplementation did not change TPC levels. Although IC50 activity was high in VB, it was not sufficient to increase the IC50 in T5 and T10 diets.
2.5. Milk analysis
2.5.1. Chemical composition
Concentrations of fat, protein, lactose and total dry extract were determined using an infrared analyzer (LactoStar Funke Gerber®). Somatic cell count (SCC) was determined using a digital counter (Ekomilk Scan Somatic Cells Analyzer®).
2.5.2. Analysis of oxidants and antioxidants
The activity of the superoxide dismutase (SOD) was determined according to the pyrogallol self-oxidation principle (inhibition in the presence of SOD). The variation in optical density was determined kinetically for two minutes at 420 nm at ten second intervals according to the methodology described by Beutler (1984). Activity was expressed as unit (U) mg/protein. Antioxidant capacity against peroxyl radicals (ACAP) concentrations were determined according to Amado et al. (2009) and results were presented as fluorescence units (FU)/mg of protein.
Levels of reactive oxygen species (ROS) in milk were analyzed using the method described by Ali et al. (1992). Aliquots of 10 μL of serum were incubated with 12 μL of dichlorofluorescein per 1 mm at 37 °C for 1 h in the dark. Fluorescence was determined using 488 nm for excitation and 520 nm for emission and the results were expressed as U DCF/mL. Levels of lipid peroxidation (LPO) were measured using the methodology proposed by Monserrat et al. (2003). Results were expressed as nmol cumene hydroperoxide (CHP)/mL.
2.6. Serum analysis
2.6.1. Biochemistry
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltransferase (GGT), total protein (TP), albumin, urea, triglycerides and cholesterol were measured using a semi-automated analyzer (BioPlus, 2000®) with commercial kits (Analisa®, Gold Analisa Diagnóstica, Belo Horizonte, Brazil). Globulin levels were calculated using the following formula: total protein level – albumin level.
2.6.2. Serum oxidant and antioxidant analysis
Determination of SOD activity was performed according to Beutler's (1984) methodology. Non-protein thiols (NPSH) were measured following the methodology of Sedlak and Lindsay (1968). The ROS levels were quantified according to Ali et al. (1992), and LPO levels were obtained following the method of Monserrat et al. (2003).
2.6.3. Enzymes of energetic metabolism
Serum creatine kinase (CK) activity was assayed based on the colorimetric method established by Hughes (1962), estimating creatine levels at a wavelength of 540 nm, and the results were expressed as nmol creatine formed/min/mg of protein. Pyruvate kinase (PK) activity was assayed according to protocol established by Leong et al. (1981) and activity was expressed as nmol pyruvate formed/min/mg of protein.
2.7. Statistical analysis
An individual ewe was considered the experimental unit for all analyses. All dependent variables were tested for normality using univariate procedure of SAS (SAS Inst. Inc., Cary, NC, USA; version 9.4) and were log transformed when needed. Then, all data were analyzed using the MIXED procedure of SAS, with Satterthwaite approximation to determine the denominator degrees of freedom for the test of fixed effects. Total milk production in five days (day 16–20), and feed conversion were tested for fixed effect of treatment using animal as random effect. All other variables were analyzed as repeated measures and tested for fixed effects of day, and treatment × day. Means were separated using PDIFF and all results were reported as LSMEANS followed by SEM. Significance was defined when P ≤ 0.05, and tendency when P > 0.05 and ≤0.10.
3. Results
3.1. Biocholine powder®
The chemical composition of the product is shown in Table 1. The commercial product had 964 g/kg of dry matter. The level of total phosphatidylcholine was 16.8 g/kg of VB, and that of phytol was 0.58 g/kg of VB. The levels of phosphatidylcholine intake per sheep/day were 0.084 g and 0.168 g for T5 and T10 groups, respectively.
3.2. Milk production
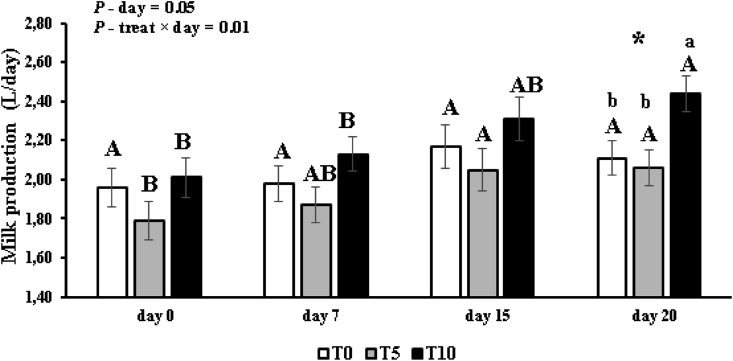
Ewes from T10 produced more milk during the experimental period (days 16–20) than that of the other treatments (Table 2). Effects of treatment × day were detected (P = 0.01) for milk production, and T10 ewes had greater production on day 20 than did T0 and T5 ewes (Figure 1). Effects of day for milk production was observed in treatments T5 and T10, characterized by an increase in production over time (Figure 1). The T5 ewes had greater (P = 0.01) feed intake compared to T0 and T10 ewes (Table 2). Sheep that consumed 10 g VB (T10) had lower feed conversion (DM intake/milk yield) than T5 and control (Table 2).
Table 2.
Feed intake, total milk production (day 16–20), productive efficiency, and feed conversion of Lacaune ewes at peak lactation supplemented with dietary vegetable biocholine.
| Variables | T0 | T5 | T10 | SEM | p-value |
|---|---|---|---|---|---|
| Milk production (L) | |||||
| Sum of day 16–20 | 10.5b | 10.3b | 12.2a | 0.11 | 0.01 |
| Mean of day 16–20 |
2.11b |
2.06b |
2.44a |
0.09 |
0.01 |
| Feed conversion | |||||
| DM intake/Milk yield | 0.93a | 1.02a | 0.78c | 0.10 | 0.01 |
Note 1: a-b Effects of treatment (line), means without a common superscript differ (P ≤ 0.05).
Note 2: T0, T5 and T10 represents 0, 5 and 10 g of Biocholine Powder®/animal/day.
Note 3: Feed intake by group (silage + concentrate) between at day 16–20: 87.2 % of DM (T0), 93.3% of DM (T5), and 84.2% of DM.
Figure 1.
Milk production of Lacaune ewes at peak lactation supplemented with dietary vegetable biocholine (VB). T0, T5 and T10 represents 0, 5 and 10 g of VB/animal/day. Asterisk (∗ - only day 20) shows treatment effect; and small letters different (a-b) showed theses effects of treatment (P ≤ 0.05). Effects of day in each of the treatments over time, where we use different capital letters (A−B) to show the difference in each treatment (P ≤ 0.05).
3.3. Composition and quality
No significant differences were detected for milk protein, fat, or lactose levels among groups (P ≥ 0.05; Table 3). However, T10 ewes had greater levels of milk total solids on day 20 than did T0 and T5 ewes (Table 3). T10 had lower SCC count on day 15 and 20 (P < 0.0001) compared to T0 ewes (Table 3), as well as we verified the effect of the day only at T10, that is, the SCC was lower on days 15 and 20 when compared to days 0. We did not find any difference between T0 and T5 for SCC (P > 0.05).
Table 3.
Milk composition and somatic cell count of Lacaune ewes at peak lactation supplemented with dietary vegetable biocholine.
| Variables1 | Treatments2 |
SEM |
P-value |
|||
|---|---|---|---|---|---|---|
| T0 | T5 | T10 | Day | Treatment × day |
||
| Protein (%) |
0.48 |
0.44 |
||||
| day 0 | 3.60 | 3.62 | 3.58 | 0.14 | ||
| day 15 | 3.78 | 3.30 | 3.52 | 0.14 | ||
| day 20 |
3.79 |
3.64 |
3.59 |
0.14 |
||
| Fat (%) |
0.56 |
0.42 |
||||
| day 0 | 4.44 | 4.38 | 4.31 | 0.27 | ||
| day 15 | 4.80 | 4.69 | 4.13 | 0.27 | ||
| day 20 |
4.14 |
4.08 |
4.55 |
0.27 |
||
| Lactose (%) |
0.81 |
0.14 |
||||
| day 0 | 5.26 | 5.37 | 5.27 | 0.21 | ||
| day 15 | 5.57 | 4.84 | 5.17 | 0.21 | ||
| day 20 |
5.16 |
5.32 |
5.23 |
0.21 |
||
| Total solids (%) |
0.19 |
0.05 |
||||
| day 0 | 13.2 | 13.2 | 12.8 | 0.19 | ||
| day 15 | 14.0 | 12.6 | 12.7 | 0.19 | ||
| day 20 |
13.1b |
13.1b |
15.6a |
0.19 |
||
| SCC1 (x103/mL) |
0.05 |
<0.0001 |
||||
| day 0 | 364.1 | 368.0 | 564.7A | 138 | ||
| day 15 | 392.6a | 250.8ab | 108.5Bb | 144 | ||
| day 20 | 436.5a | 209.9ab | 101.1Bb | 151 | ||
a-bEffect of the interaction between treatment versus day (line), the averages followed by different lowercase letters differ from each other (P ≤ 0.05).
A,BDay effect (column), the averages followed by different capital letters differ from each other (P ≤ 0.05).
Somatic cell count - SCC.
T0, T5 and T10 represents 0, 5 and 10 g of Biocholine Powder®/animal/day.
T5 and T10 ewes had lower LPO levels in milk on day 20 compared to T0 ewes (Table 4). Effects of day and interaction (treatment × day) were detected for milk concentration of ROS (i.e., at T5 and T10, ewes had lower concentrations on day 20 when compared to T0 ewes; Table 4). Moreover, T5 and T10 ewes had greater milk ACAP levels on day 20 than did T0 ewes (P < 0.01; Table 4). T0 ewes resulted in lower SOD activity on day 15 that was increased on day 20 and was similar to that of day 0 (Table 4). A day effect was observed for LPO, ROS, ACAP, and SOD in milk (P < 0.05). In particular, there were lower LPO levels and greater ACAP levels over time in milk sheep from T5 and T10. In all groups over time, there were reduced levels of ROS (Table 4); this was similar to what occurred with SOD activity in treatments T0 and T5. However, the consumption of the additive in the highest dose (T10) gave higher SOD activity when compared to the 20th as also the 15th (Table 4).
Table 4.
Milk oxidant/antioxidant status of Lacaune ewes at peak lactation supplemented with dietary Biocholine Powder®.
| Variables1 | Treatments2 |
SEM |
P-value |
|||
|---|---|---|---|---|---|---|
| T0 | T5 | T10 | Day | Treatment × day | ||
| LPO (nmol/mL) |
<0.0001 |
0.05 |
||||
| day 0 | 771 | 757A | 643A | 145 | ||
| day 15 | 660 | 758A | 713A | 145 | ||
| day 20 |
582a |
218Bb |
240Bb |
145 |
||
| ROS (U DCF/mg protein) |
<0.0001 |
<0.0001 |
||||
| day 0 | 2.12A | 2.03A | 1.97A | 0.25 | ||
| day 15 | 1.61AB | 1.15B | 1.16B | 0.27 | ||
| day 20 |
1.40Ba |
0.45Cb |
0.67Bb |
0.27 |
||
| ACAP (U.F mg/μmol/mL) |
<0.0001 |
<0.0001 |
||||
| day 0 | 0.70 | 0.73C | 0.71C | 0.07 | ||
| day 15 | 0.59 | 0.92B | 0.90B | 0.07 | ||
| day 20 |
0.61b |
1.07Aa |
1.15Aa |
0.07 |
||
| SOD (U SOD/mg protein) |
<0.0001 |
<0.0001 |
||||
| day 0 | 4.51A | 4.30A | 3.94A | 0.31 | ||
| day 15 | 3.33B | 3.97A | 3.45B | 0.31 | ||
| day 20 | 3.15Bb | 2.96Bb | 4.30Aa | 0.31 | ||
a-bEffect of the interaction between treatment versus day (line), the averages followed by different lowercase letters differ from each other (P ≤ 0.05).
A,BDay effect (column), the averages followed by different capital letters differ from each other (P ≤ 0.05).
Lipid peroxidation (LPO), reactive oxygen species (ROS), antioxidants non-protein thiols (NPSH) and superoxide dismutase (SOD).
T0, T5 and T10 represents 0, 5 and 10 g of Biocholine Powder®/animal/day.
3.4. Serum biochemistry and oxidant/antioxidant status
There was no interaction (treatment x day) and treatment effect for serum concentration of glucose, total protein, albumin, cholesterol, triglycerides, and urea, as well as activities of AST, ALT, GGT, CK and PK (Tables 4 and 5). Effects of the day were observed for glucose, albumin, triglycerides, and cholesterol levels in the three treatments; in particular, there were increases over time for albumin, triglycerides, and cholesterol in sheep serum. A day effect for urea levels occurred only for sheep that consumed VB (T5 and T10); that is, in the T5 group, there was an increase from day 15 when compared to days 0 and 20 (Table 5); and group T10 had lower urea levels on day 20 when compared to days 0 and 15 of the experiment. GGT activity decreased over time in the blood of sheep in groups T5 and T10 (Table 6). The other liver enzymes and energy metabolism enzymes showed no effect of the day (P > 0.05; Table 6).
Table 5.
Serum biochemistry (metabolism) of peak lactation sheep Lacaune supplemented with dietary vegetable biocholine
| Variables | Treatments1 |
SEM |
P-value |
|||
|---|---|---|---|---|---|---|
| T0 | T5 | T10 | Day | Treatment × day | ||
| Glucose (mg/dL) |
<0.0001 |
0.95 |
||||
| day 0 | 43.3B | 42.0B | 42.0B | 2.31 | ||
| day 15 | 55.8A | 58.7A | 59.0A | 2.31 | ||
| day 20 |
45.1B |
42.3B |
43.0B |
2.31 |
||
| Total Protein (g/dL) |
0.85 |
0.97 |
||||
| day 0 | 6.66 | 6.67 | 6.66 | 0.17 | ||
| day 15 | 6.39 | 6.77 | 6.88 | 0.17 | ||
| day 20 |
6.59 |
6.62 |
6.52 |
0.17 |
||
| Albumin (g/dL) |
<0.0001 |
0.84 |
||||
| day 0 | 2.87AB | 2.90AB | 2.87AB | 0.11 | ||
| day 15 | 2.53B | 2.63B | 2.65B | 0.11 | ||
| day 20 |
3.01A |
3.27A |
3.06A |
0.11 |
||
| Globulin (g/dL) |
<0.0001 |
0.05 |
||||
| day 0 | 3.79B | 3.77B | 3.79B | 0.06 | ||
| day 15 | 3.76Bb | 4.14Aa | 4.22Aa | 0.06 | ||
| day 20 |
3.37Ab |
3.37Bb |
3.71Ba |
0.06 |
||
| Cholesterol (mg/dL) |
<0.0001 |
0.35 |
||||
| day 0 | 48.9B | 48.6B | 51.3B | 3.52 | ||
| day 15 | 60.6A | 71.2A | 67.2A | 3.92 | ||
| day 20 |
59.4A |
63.9A |
55.9B |
3.52 |
||
| Triglycerides (mg/dL) |
<0.0001 |
0.11 |
||||
| day 0 | 13.5B | 14.9B | 13.2B | 1.15 | ||
| day 15 | 15.4B | 14.9B | 12.9B | 1.17 | ||
| day 20 |
20.9A |
19.8A |
18.3A |
1.19 |
||
| Urea (mg/dL) |
<0.0001 |
0.17 |
||||
| day 0 | 32.6 | 34.5B | 35.6A | 2.80 | ||
| day 15 | 38.0 | 43.2A | 39.2A | 2.94 | ||
| day 20 | 32.6 | 30.8B | 23.4B | 2.81 | ||
a-bEffect of the interaction between treatment versus day (line), the averages followed by different lowercase letters differ from each other (P ≤ 0.05).
A,BDay effect (column), the averages followed by different capital letters differ from each other (P ≤ 0.05).
T0, T5 and T10 represents 0, 5 and 10 g of Biocholine Powder®/animal/day.
Table 6.
Hepatic enzymes and the energetic metabolism of peak lactation sheep Lacaune supplemented with dietary vegetable biocholine.
| Variables1 | Treatments2 |
SEM | P-value |
|||
|---|---|---|---|---|---|---|
| T0 | T5 | T10 | Day | Treatment × day | ||
| AST (U/L) |
0.27 |
0.61 |
||||
| day 0 | 110 | 107 | 101 | 7.17 | ||
| day 15 | 114 | 106 | 102 | 7.17 | ||
| day 20 |
99.7 |
116 |
102 |
7.50 |
||
| ALT (U/L) |
15.0 |
15.5 |
16.0 |
0.39 |
0.11 |
0.35 |
| day 0 | 14.8 | 14.8 | 15.4 | 0.70 | ||
| day 15 | 14.2 | 15.6 | 15.5 | 0.70 | ||
| day 20 |
16.0 |
16.6 |
15.6 |
0.70 |
||
| GGT (U/L) |
0.01 |
0.77 |
||||
| day 0 | 106 | 101AB | 97.7AB | 5.10 | ||
| day 15 | 106 | 111A | 105A | 5.10 | ||
| day 20 |
101 |
98.0B |
89.1B |
5.10 |
||
| CK (pmol creatine formed/min/mg of protein) |
0.12 |
0.48 |
||||
| day 0 | 1.70 | 1.67 | 1.70 | 0.09 | ||
| day 15 | 1.81 | 1.64 | 1.73 | 0.09 | ||
| day 20 |
1.47 |
1.48 |
1.73 |
0.09 |
||
| PK (pmol pyruvate formed/min/mg protein) |
8.71 |
7.96 |
7.96 |
0.36 |
0.38 |
0.13 |
| day 0 | 8.14 | 8.97 | 8.47 | 0.55 | ||
| day 15 | 8.92 | 7.06 | 7.77 | 0.55 | ||
| day 20 | 9.09 | 7.87 | 7.65 | 0.55 | ||
a-bEffect of the interaction between treatment versus day (line), the averages followed by different lowercase letters differ from each other (P ≤ 0.05).
A,BDay effect (column), the averages followed by different capital letters differ from each other (P ≤ 0.05).
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltransferase (GGT), creatine kinase (CK), and pyruvate kinase (PK).
T0, T5 and T10 represents 0, 5 and 10 g of Biocholine Powder®/animal/day.
An effect of the day was observed for globulin levels in the three groups, with emphasis on the reduction of globulins in T0 on day 20 (Table 5). The serum concentration of globulin in T5 and T10 ewes was greater on day 15; however, increased globulin content was observed in T10 than in T0 and T5 groups on day 20 (Table 5).
Interaction occurred (treatment x day) for serum levels of LPO and ROS, i.e. the T5 and T10 ewes had lower serum levels for LPO and ROS on day 15 and 20 than did T0 ewes (Table 7). Effects of the day were observed for LPO and ROS levels in the serum of sheep in groups T5 and T10; this was characterized by a reduction in the concentration of these two parameters (Table 7).
Table 7.
Serum oxidant/antioxidant status of Lacaune ewes at peak lactation supplemented with dietary vegetable biocholine.
| Variables1 | Treatments2 |
SEM |
P-value |
|||
|---|---|---|---|---|---|---|
| T0 | T5 | T10 | Day | Treatment × day | ||
| LPO (nmol/mL) |
<0.0001 |
<0.0001 |
||||
| day 0 | 191 | 206.4A | 204.8A | 17.1 | ||
| day 15 | 192a | 59.4Bb | 58.2Bb | 17.1 | ||
| day 20 |
165a |
88.8Bb |
78.0Bb |
18.1 |
||
| ROS (U DCF/mg protein) |
<0.0001 |
<0.0001 |
||||
| day 0 | 1.17 | 1.25A | 1.38A | 0.10 | ||
| day 15 | 1.35a | 0.44Bb | 0.29Bb | 0.11 | ||
| day 20 |
1.40a |
0.48Bb |
0.45Bb |
0.10 |
||
| NPSH (μmol/mL) |
<0.0001 |
<0.0001 |
||||
| day 0 | 1.93A | 1.88B | 1.92B | 0.07 | ||
| day 15 | 1.91A | 1.84B | 1.79C | 0.07 | ||
| day 20 |
1.71Bc |
2.03Ab |
2.21Aa |
0.07 |
||
| SOD (U SOD/mg protein) |
0.42 |
0.38 |
||||
| day 0 | 5.88 | 5.62 | 5.81 | 0.3 | ||
| day 15 | 5.82 | 5.49 | 5.22 | 0.3 | ||
| day 20 | 5.64 | 6.06 | 5.87 | 0.3 | ||
a-bEffect of the interaction between treatment versus day (line), the averages followed by different lowercase letters differ from each other (P ≤ 0.05).
A,BDay effect (column), the averages followed by different capital letters differ from each other (P ≤ 0.05).
Lipid peroxidation (LPO), reactive oxygen species (ROS), antioxidants non-protein thiols (NPSH) and superoxide enzyme dismutase (SOD).
T0, T5 and T10 represents 0, 5 and 10 g of Biocholine Powder®/animal/day.
T5 and T10 ewes had greater NPSH serum concentrations on day 20 than did T0 ewes (P < 0.01; Table 7). Effects of the day were observed for NPSH levels in the sheep serum from the three groups (P < 0.05), these findings were inverse, because there was a reduction over time in the T0 ewes and an increase during the same period in groups T5 and T10. Interaction of treatment by day and day effect were not significant (P ≥ 0.05) for serum concentration of SOD (Table 7).
4. Discussion
Milk production is directly associated with the amount of feed available to sheep, as well as to the quality and availability of nutrients in the feed. In a study, where encapsulated choline chloride added to cow feed, there was an increase in milk production in supplemented animals, without changing milk fat and protein levels, as well as plasma glucose and cholesterol levels (Pinotti et al., 2003). These findings were similar to those observed in the present study. In addition, we found higher levels of total solids in the milk of sheep that ingested the highest dose of VB, a positive effect because milk is primarily intended for the production of processed foods. Nevertheless, the reasons that led to increased total solids remain to be investigated. Encapsulated choline supplements in multiparous cows promoted increased milk production in animals receiving 30 g per day, also without changing the milk composition (Xu et al., 2006), as in our study. According to the authors, choline supplementation in the diets of high-yield dairy cows is essential to maintain milk yield and quality, because choline deficiency may be a limiting factor for production (Pinotti et al., 2002; Baldi and Pinotti, 2006). In our study, dairy ewes at peak production in summer increased milk production when supplemented with VB.
In the present study, serum hepatic biochemical variables did not respond to supplementation when we evaluated the effect of treatment and treatment x day, as previously described by Michailoff et al. (2013). Nevertheless, a day effect was observed in sheep that consumed VB (T5 and T10), because GGT activity decreased with the time in these groups. In a study, the addition of encapsulated choline in Holstein cow diets decreased blood ketone concentrations (Michailoff et al., 2013). Supplementation of protected choline to pregnant sheep reduced serum ketone levels, in addition to decreasing clinical and subclinical cases of ketosis (Michailoff et al., 2017). The reduced susceptibility to ketosis in early lactating dairy cows supplemented with encapsulated choline may occur due to improvements in hepatic fat export (Zom et al., 2011). Hepatoprotective effects in ewes (Alba et al., 2020) supplemented with VB was observed. Therefore, we cannot exclude potent liver protection provided by VB, because we used only indirect variables (i.e., liver enzyme levels). In addition, the effect of day on urea levels may indicate that commercial product increased protein catabolism, because synthesis of albumin by the liver also increased.
In our study, ewes receiving VB showed greater milk production, possibly related to greater passage of phosphatidylcholine through the rumen and its absorption in the intestine. According to the literature, VB has choline conjugates such as phosphatidylcholine that display natural resistance to ruminal degradation (Godínez-Cruz et al., 2015). Another study in sheep fed with VB reported high bioavailability; productive results of animals supplemented with natural commercial product were similar to those receiving encapsulated choline chloride (Crosby et al., 2017). This information was what stimulated this study, thereby helping to explain our positive results in terms of milk production and quality.
The decrease in SCC in animals supplemented with the highest dose of VB (T10) was a beneficial result of this study; this is because low SCC values are associated with improved mammary gland health that is directly related to better quality milk produced (Bozo et al., 2013). Recent in vitro research has shown that VB has antimicrobial potential against E. coli (Dazuk et al., 2020, in press). Animals with subclinical mastitis produce lower volumes of milk with higher cellularity as well as higher levels of oxidants (Alba et al., 2019b), factors that may affect the productive chain (microbiological quality and shelf life of milk product) (Coelho et al., 2014). Due to the fact that choline participates in cell formation, cell signaling and tissue integrity (Zeisel and Blusztajn, 1994; Zeisel, 1988), we believe that animals receiving VB may have stronger cell structures, with consequent reduction in desquamation of the udder secretory epithelium, thereby reducing SCCs.
The bioavailability of choline and derivatives is important for maintaining cellular integrity. Choline deficiency increases levels of reactive oxygen species and lipid peroxidation that in turn causes necrosis of the convoluted renal tubules (Ossani et al., 2007). Repetto et al. (2010) also found increased lipid peroxidation and reduced antioxidant levels in the serum of choline-deficient rats, while in the brain, lipid peroxidation levels increased by more than 300%. As a result, the animals developed hepatic steatosis, tubular and glomerular necrosis in addition to inflammation and necrosis in the heart after 7 days of deficiency. In our study, supplementation with VB promoted increases in NPSH and ACAP levels, as well as reduced ROS and LPO levels; a similar result was evident in fish (Baldissera et al., 2019; Souza et al., 2020).
5. Conclusion
VB-supplementation in the diets of sheep at peak lactation increased antioxidant levels in serum as well as in milk, reducing oxidant levels and lipid peroxidation. The addition of commercial product to feed had a positive effect on milk production, and decreased somatic cell counts in milk. We conclude that VB supplementation in the diets of sheep improves animal health. In general, 10 g of VB/animal/day is an excellent alternative for Lacaune dairy sheep.
Declarations
Author contribution statement
Davi F. Alba, Aleksandro S. Da Silva: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Karoline Leal, Marily H. Cunha, Hiam Marcon: Performed the experiments; Wrote the paper.
Gilneia da Rosa, Carine F. Souza, Matheus D. Baldissera, Renata L. Kavalek: Analyzed and interpreted the data; Wrote the paper.
Claiton A. Zotti, Aniela P. Kempka, Marcelo Vedovatto: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by CAPES (Brazil), CNPq (Brazil), and UDESC (PROMOP - Santa Catarina, Brazil).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We also thank Cabanha Chapecó for making the animals and the facility available for this study.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adler J.H. Theoretical quantitative approach to the mechanism of hypoglycemic ketosis in ruminants. J. Theor. Biol. 1970;28(1):101–109. doi: 10.1016/0022-5193(70)90066-4. [DOI] [PubMed] [Google Scholar]
- Alba D.F., Campigotto G., Cazarotto C.J., Santos D.S., Gebert R.R., Reis J.H., Souza C.F., Baldissera M.D., Gindri A.L., Kempka A.P., Palmer E.A., Vedovatto M., Da Silva A.S. Use of grape residue flour in lactating dairy sheep in heat stress: effects on health, milk production and quality. J. Therm. Biol. 2019;82:197–205. doi: 10.1016/j.jtherbio.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Alba D.F., Rosa G., Hanauer D., Saldanha T.F., Souza C.F., Baldissera M.D., Santos D.S., Piovezan A.P., Girardini L.K., Da Silva A.S. Subclinical mastitis in Lacaune sheep: causative agents, impacts on milk production, milk quality, oxidative profiles and treatment efficacy of ceftiofur. Microb. Pathog. 2019;137:103732. doi: 10.1016/j.micpath.2019.103732. [DOI] [PubMed] [Google Scholar]
- Alba D.F., Favaretto J.A., Marcon H., Bianchi A.E., Vedovatto M., Da Silva A.S. Vegetable biocholine supplementation in pre- and postpartum Lacaune sheep: effects on animal health, milk production and quality. Small Rumin. Res. 2020;190:106165. [Google Scholar]
- Ali S.F., Lebel C.P., Bondy S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicologia. 1992;13:637–648. [PubMed] [Google Scholar]
- Amado L.L., Garcia M.L., Ramos P.B., Freitas R.F., Zafalon B., Ferreira J.L.R., Yunes J.S., Monserrat J.M. A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: application to evaluate microcystins toxicity. Sci. Total Environ. 2009;407:2115–2123. doi: 10.1016/j.scitotenv.2008.11.038. [DOI] [PubMed] [Google Scholar]
- AOAC International . 15th ed. Association of Analytical Chemist; Virginia: 1990. Association of Official Analytical Chemistry. Official Methods of Analysis; p. 287. [Google Scholar]
- Baird G.D. Aspects of ruminant intermediary metabolism in relation to ketosis. Biochem. Soc. Trans. 1977;5(3):819–827. doi: 10.1042/bst0050819. [DOI] [PubMed] [Google Scholar]
- Baldi A., Pinotti L. Choline metabolism in high-producing dairy cows: metabolic and nutritional basis. Can. J. Anim. Sci. 2006;86(2):207–212. [Google Scholar]
- Baldissera M.D., Souza C.F., Baldisserotto B., Zimmerc F., Paiano D., Petrolli T.G., Da Silva A.S. Vegetable choline improves growth performance, energetic metabolism, and antioxidant capacity of fingerling Nile tilapia (Oreochromis niloticus) Aquaculture. 2019;501:224–229. [Google Scholar]
- Berchielli T.T., Pires A.V., Oliveira S.G. In: Ruminant Nutrition. 2nd ed. Jaboticabal S.P., editor. FUNEP; 2011. p. 616. [Google Scholar]
- Bernabucci U., Ronchi B., Lacetera N., Nardone A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005;88(6):2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2. [DOI] [PubMed] [Google Scholar]
- Beutler E. A Manual of Biochemical Methods. Grune & Stratton; Philadelphia: 1984. Superoxide dismutase. Red cell metabolism; pp. 83–85. [Google Scholar]
- Bozo G.A., Alegro L.C.A., Silva L.C., Santana1 E.H.W., Okano W., Silva L.C.C. Suitability of somatic cell count and total bacterial count in raw refrigerated milk to legislation standards. Arq. Bras. Med. Vet. Zootec. 2013;65(2):589–594. [Google Scholar]
- Coelho K.O., Mesquita A.J., Machado P.F., Lage M.E., Meyer P.M., Reis A.P. The effect of somatic cell count on yield and physico-chemical composition of Mozzarella cheese. Arq. Bras. Med. Vet. Zootec. 2014;66(4):1260–1268. [Google Scholar]
- Crosby M., Mendoza-Martinez G.D., Relling A., Vazquez V.A., Lee-Rangel H.A., Martinez J.A., Oviedo M. Influence of supplemental choline on milk yield, fatty acid profile, and postpartum weight changes in suckling ewes. J. Dairy Sci. 2017;100(2):125. [Google Scholar]
- Cunha M.G., Alba D.F., Leal K.W., Marcon H., Souza C.F., Baldissera M.D., Da Silva A.S. Inclusion of pepper extract containing capsaicin in the diet of ewes in the mid-lactation period: effects on health, milk production, and quality. Res. Soc. Dev. 2020;9 [Google Scholar]
- Dazuk V., Boiago M.M., Da Silva A.S. Vegetable biocholine as a hepatoprotectant in laying hens feed with diet contaminated with aflatoxin B1. World Mycotoxin J. 2020 in press. [Google Scholar]
- FAO Milk and dairy products in human nutrition. Food and agriculture organization of the united nations. 2013. http://www.fao.org/3/i3396e/i3396e.pdf Disponível em. Acesso em 14 set. 2019. [PubMed]
- Fernandes L.B., Franzolin R., Franco A.V.M., Carvalho G. Organic additives in concentrate supplementation of beef cattle grazing. Rev. Bras. Saúde Prod. Animal. 2008;9(2):231–238. [Google Scholar]
- Godínez-Cruz J., Cifuentes-López O., Cayetano J., Lee-Rangel H., Mendoza G., Vázquez A., Roque A. Effect of choline inclusion on lamb performance and meat characteristics. J. Anim. Sci. 2015;93(3):766. [Google Scholar]
- Hughes B.P. A method for estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin. Chim. Acta. 1962;7:597–603. doi: 10.1016/0009-8981(62)90137-7. [DOI] [PubMed] [Google Scholar]
- IBGE . 2018. Number of herds. Instituto Brasileiro de Geografia e Estatística.https://sidra.ibge.gov.br/tabela/3939#resultado Disponível em: Acesso em 14 set. 2019. [Google Scholar]
- Jaguezeski A.M., Perin G., Bottari N.B., Wagner R., Fagundes M.B., Schetinger M.R.C., Morsch V.M., Da Silva A.S. Addition of curcumin to the diet of dairy sheep improves health, performance and milk quality. Anim. Feed Sci. Technol. 2018;246:144–157. [Google Scholar]
- Kupke I.R., Zeugner S. Quantitative high-performance thin-layer chromatography of lipids in plasma and liver homogenates after direct application of 0.5-microliter samples to the silica-gel layer. J. Chromatogr. 1978;146(2):261–271. doi: 10.1016/s0378-4347(00)81892-7. [DOI] [PubMed] [Google Scholar]
- Leong S.F., Lai J.C., Lim L., Clark J.B. Energy-metabolizing enzymes in brain regions of adult and aging rats. J. Neurochem. 1981;37:1548–1556. doi: 10.1111/j.1471-4159.1981.tb06326.x. [DOI] [PubMed] [Google Scholar]
- Li Z., Vance D.E. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008 Jun;49(6):1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- Li Z., Agellon L.B., Vance D.E. Phosphatidylcholine homeostasis and liver failure. J. Biol. Chem. 2005;280(45):37798–37802. doi: 10.1074/jbc.M508575200. [DOI] [PubMed] [Google Scholar]
- Mader T.L., Davis M.S., Brown-Brandl T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006;84:712–719. doi: 10.2527/2006.843712x. [DOI] [PubMed] [Google Scholar]
- Mehedint M.G., Zeisel S.H. Choline's role in maintaining liver function: new evidence for epigenetic mechanisms. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16(3):339–345. doi: 10.1097/MCO.0b013e3283600d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailoff A., Dall Agnol D.D., Balistieri F.S., Mores F., Ferreira R., Bragança J.F., Rocha J.F.X., Rocha R.X. Desempenho produtivo e status energético em vacas leiteiras suplementadas com colina protegida. Rev. Acad. Ciênc. Agr. Amb. 2013;11(4):367–372. [Google Scholar]
- Michailoff A.A.M., Agnol D.D.D., Bianchi A.E., Balistieri F.S., Fiorentin E.L., Rocha R.X., Bragança J.F.M. Rumen protected choline and its effect on the prevention of pregnancy toxemia in dairy ewes. Vet. Zootec. 2017;24(1):144–150. [Google Scholar]
- Monserrat J.M., Geracitano L.A., Pinho G.L.L., Vinagre T., Faleiros M., Alciati J.C., Bianchini A. Determination of lipid peroxides in invertebrates tissues using the Fe (III) xylenol orange complex formation. Arch. Environ. Contam. Toxicol. 2003;45:177–183. doi: 10.1007/s00244-003-0073-x. [DOI] [PubMed] [Google Scholar]
- Ossani G., Dalghi M., Repetto M. Oxidative damage lipid peroxidation in the kidney of choline-deficient rats. Front. Biosci. J. Vis. Literacy. 2007;12:1174–1183. doi: 10.2741/2135. [DOI] [PubMed] [Google Scholar]
- Pavic C., Antunac N., Mioč B., Ivanković A., Havranek J.L. Influence of stage of lactation on the chemical composition and physical properties of sheep milk. Czech J. Anim. Sci. 2002;47(2):80–84. [Google Scholar]
- Pinotti L., Baldi A., Dell’Orto V. Comparative mammalian choline metabolism with emphasis on the high-yielding dairy cow. Nutr. Res. Rev. 2002;15:315–331. doi: 10.1079/NRR200247. [DOI] [PubMed] [Google Scholar]
- Pinotti L., Baldi A., Politis I., Rebucci R., Sangalli L., Dell’Orto V. Rumen-protected choline administration to transition cows: effects on milk production and vitamin E status. J. Vet. Med. Ser. A. 2003;50:18–21. doi: 10.1046/j.1439-0442.2003.00502.x. [DOI] [PubMed] [Google Scholar]
- Repetto M.G., Ossani G., Monserrat A.J., Boveris A. Oxidative damage: the biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 2010;88:143–149. doi: 10.1016/j.yexmp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Guerrero V., Lizarazo A.C., Ferraro, Suárez S.N., Miranda L.A., Mendoza G.D. Effect of herbal choline and rumen-protected methionine on lamb performance and blood metabolites. S. Afr. J. Anim. Sci. 2018;48(3):427–434. [Google Scholar]
- Rukkwamsuk T., Kruip T.A.M., Wensing T. Relationship between overfeeding and overconditioning in the dry period and the problems of high producing dairy cows during the postparturient period. Vet. Q. 1999;21(3):71–77. doi: 10.1080/01652176.1999.9694997. [DOI] [PubMed] [Google Scholar]
- Santos D.S., Klauck V., Campigotto G., Alba D.F., Reis J.H., Gebert R.R., Souza C.F., Baldissera M.D., Schogor A.L.B., Santos I.D., Wagner R., Vedovatto M., Da Silva A.S. Benefits of the inclusion of açai oil in the diet of dairy sheep in heat stress on health and milk production and quality. J. Therm. Biol. 2019;84:250–258. doi: 10.1016/j.jtherbio.2019.07.007. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total protein-bound, and nonprotein sulfhydryl groups in tissue with ellman’s reagent. Anal. Biochem. 1968;25:1192–1205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Souza C.F., Baldissera M.D., Baldisserotto B., Petrolli T.G., Micottida E., Zanette G.R.A., Da Silva A.S. Dietary vegetable choline improves hepatic health of Nile tilapia (Oreochromis niloticus) fed aflatoxin-contaminated diet. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;227 doi: 10.1016/j.cbpc.2019.108614. [DOI] [PubMed] [Google Scholar]
- Ticiani E., Sandri E.C., Souza J., Batiste F., Oliveira D.E. Lactation persistency and milk composition in Lacaune and East Friesian dairy ewes. Ciência Rural. 2013;43(9):1650–1653. [Google Scholar]
- Valencia Narváez M.Á. Universidad Nacional de Trujillo, Peru; 2019. Efecto de la biocolina sobre calidad de leche y comportamiento productivo pre y postparto en vacas lecheras. Tesis para optar el título de Ingeniero Zootecnista.http://www.dspace.unitru.edu.pe/handle/UNITRU/13313 Disponível em: Acesso em 16 set. 2019. [Google Scholar]
- Van Soest P.J. 2 ed. Cornell University Press; Ithaca: 1994. Nutritional Ecology of the Ruminant; p. 476p. [Google Scholar]
- Walker A.K. 1-Carbon cycle metabolites methylate their way to fatty liver. Trends Endocrinol. Metabol. 2017;28(1):63–72. doi: 10.1016/j.tem.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W.P., Conrad H.R., Pierre N.R. St. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed Sci. Technol. 1992;39:95–110. [Google Scholar]
- Wirtz K.W. Phospholipid transfer proteins. Annu. Rev. Biochem. 1991;60(13):73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- Xu G., Ye J.A., Liu J., Yu1 Y. Effect of rumen-protected choline addition on milk performance and blood metabolic parameters in transition dairy cows. Asian-Australas. J. Anim. Sci. 2006;19(3):390–395. [Google Scholar]
- Zeisel S.H. Vitamin-like molecules: choline. In: Shils M., Young V., editors. Modern Nutrition in Health and Disease. Lea & Febiger; Philadelphia: 1988. pp. 440–452. [Google Scholar]
- Zeisel S.H., Blusztajn J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Zom R.L.G., Van Baal J., Goselink R.M.A., Bakker J.A., Veth M.J., Vuuren A.M. Effect of rumen-protected choline on performance, blood metabolites, and hepatic triacylglycerols of periparturient dairy cattle. J. Dairy Sci. 2011;94(8):4016–4027. doi: 10.3168/jds.2011-4233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.