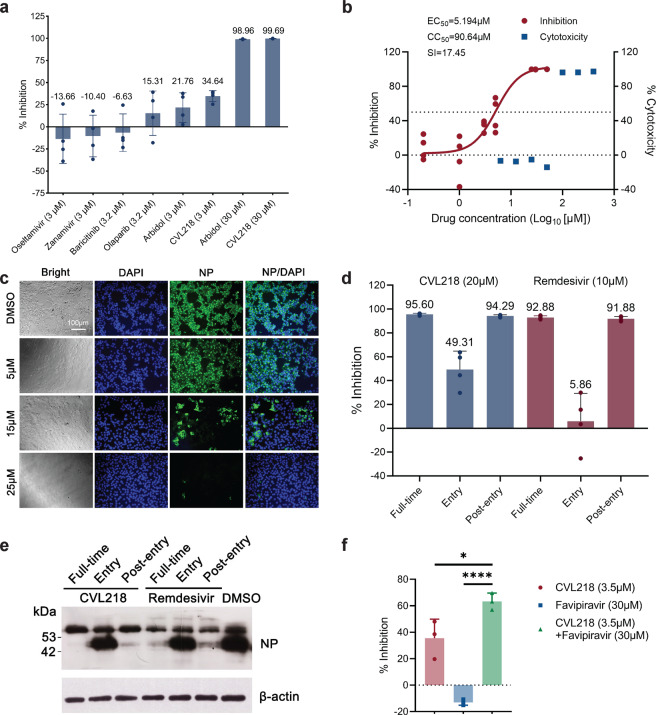
Fig. 2.
The in vitro anti-SARS-CoV-2 activities of the tested drugs in Vero E6 cells. a The preliminary in vitro antiviral activities of oseltamivir at 3 μM, zanamivir at 3 μM, baricitinib at 3.2 μM, olaparib at 3.2 μM, arbidol at 3 μM and 30 μM, and CVL218 at 3 μM and 30 μM, respectively, were detected in Vero E6 cells infected with SARS-CoV-2 at an MOI of 0.05. The viral yield in the cell supernatant was then quantified by qRT-PCR. Results are shown as mean ± SD over four replicates. b The concentration-dependent inhibition curve of CVL218 against SARS-CoV-2 replication and its cytotoxicity results. Viral infection and drug treatment at different concentrations were performed as mentioned above. Cytotoxicity of CVL218 to Vero E6 cells was measured by the CCK8 assays. c Visualization of virus nucleoprotein (NP) expression of the infected cells upon treatment of CVL218 at 48 h post the SARS-CoV-2 infection using fluorescence microscopy. d Time-of-addition results on the inhibition of CVL218 and remdesivir against SARS-CoV-2 in vitro. The viral inhibitory activities of CVL218 and remdesivir were measured at “full-time”, “entry”, and “post-entry” stages, respectively. Results are shown as mean ± SD over four replicates. e Virus NP expression in the infected cells upon the treatment of CVL218 and remdesivir was analyzed by western blot. f In vitro inhibitory activities against SARS-CoV-2 replication of favipiravir (30 μM), CVL218 (3.5 μM) and a combination of both drugs (30 μM favipiravir + 3.5 μM CVL218). The concentrations were selected according to the EC25 values of individual drugs against SARS-CoV-2 in vitro. Viral infection and drug treatment were performed as mentioned above. Results are shown as mean ± SD over three replicates, and the significances were measured by p-values from t-tests. * and **** stand for p-value < 0.05 and p-value < 0.0001, respectively