Figure 3.
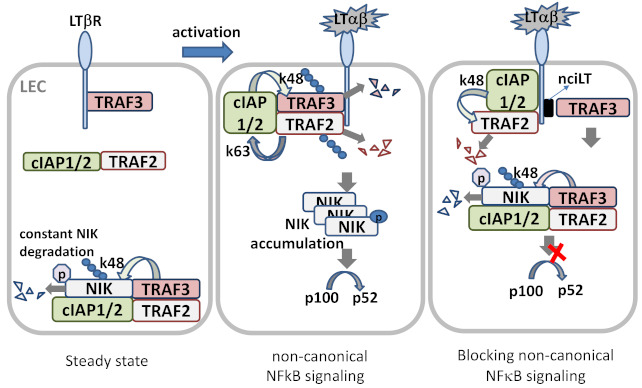
LTβR signaling in LEC. In steady state, newly synthesized NIK is rapidly bound by TRAF3 and targeted to the TRAF-cIAPs E3 ubiquitin ligase complex for K48-polyubiquitination and proteasomal degradation, where TRAF2 bridges TRAF3 and cIAPs. Low level NIK is unable to process p100 under normal conditions. In ligand (LTαβ or LIGHT)-stimulated LECs, the TRAF-cIAPs complex is recruited to the LTβR where cIAP1/2 is activated by TRAF2-mediated K63 ubiquitination, and the activated cIAP1/2 then targets TRAF3 for K48 ubiquitination and degradation. With the lack of TRAF3, de novo synthesized NIK accumulates and is activated via trans-phosphorylation. NIK then activates IKKα, leading to p100 processing and nuclear translocation of RelB/p52. Masking the TRAF3-binding site of LTβR by permeable blocking peptide nciLT leads to TRAF3 targeting NIK for degradation, and hence p100 processing is blocked.
