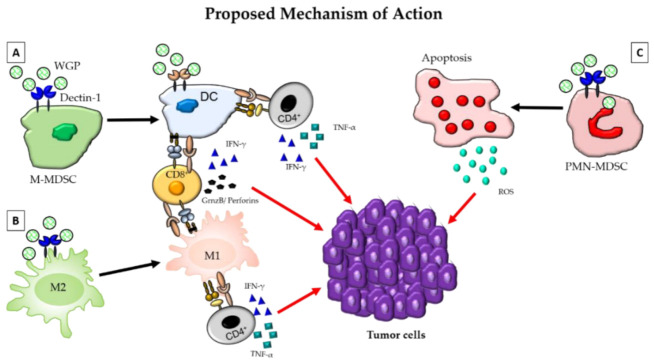
Figure 6.
The modulation of the immune cells in the tumor microenvironment by β-glucan. β-glucan binds to the Dectin-1 receptors expressed on cells of the myeloid lineage and is then phagocytosed. In (A), β-glucan can be seen binding to Dectin-1 on an M-MDSC. Binding to the M-MDSC will cause the M-MDSC to switch from a suppressive phenotype to a DC phenotype that can act as an APC. This dendritic cell (DC) will then activate CD4+ and CD8+ T-cells, where CD4+ T-cells will secrete pro-inflammatory cytokines, such as TNFα and IFN-γ, and CD8+ T-cells will secrete Granzyme B, perforins, and IFN-γ. The secretion of these pro-inflammatory cytokines by CD4+ and CD8+ T-cells will lead to the destruction of tumor cells. β-Glucan induces the polarization of suppressive M2 macrophages (B) into inflammatory M1 macrophages. M1 macrophages will then activate Th1 type T-cells, leading to damage to the tumor cells through the secretion of pro-inflammatory cytokines by CD4+ and CD8+ T-cells. Finally, in (C), β-glucan will bind to the Dectin-1 receptor on polymorphonuclear (PMN)-MDSCs and cause apoptosis of the cell. As the cell undergoes apoptosis, it will produce ROS that will ultimately target the tumor cells, leading to tumor cell death. Overall, these mechanisms together convert a suppressive tumor microenvironment (TME) to an inflammatory TME that has a greater potential to induce the killing of tumors. From Geller, Shrestha, and Yan (2019) [109].