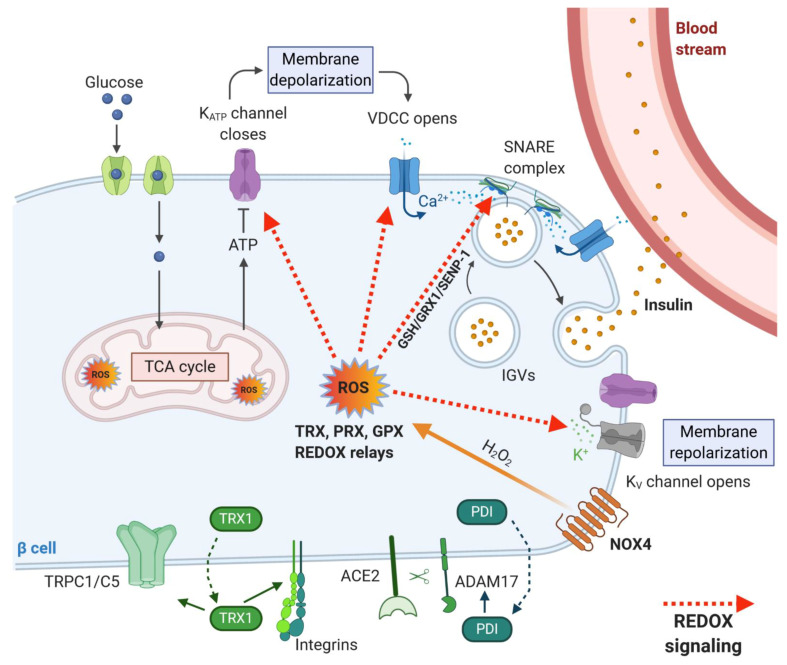
Figure 4.
Redox targets within insulin secretory pathway and proteins involved in extracellular redox signaling. Glucose entry through GLUT1 in humans or GLUT2 in rodents causes an increase in ATP/ADP ratio and elevation of ROS production in the cytoplasm (mediated by NOX4). Subsequently, the KATP channel closes, which causes depolarization of the plasma membrane, the opening of voltage-dependent calcium channels (VDCC) and Ca2+ entry into the cells. Ca2+ is required for insulin granule vesicles (IGV) trafficking, attachment and fusion with the plasma membrane (mediated by SNARE proteins) and finally, secretion of insulin from the cell. Repolarization of the membrane is mediated via voltage-gated K+ channels (KV). These repeating cycles lead to a high-frequency of action potential spikes and synchronized oscillations in the cytosolic Ca2+. ROS are utilized by redox-regulated proteins TRX, GPX and PRX, which can either neutralize the ROS action in the long-term or transform acute redox signal via redox relays. Targets of redox signaling involve KATP channels, VDCC, KV channels and proteins involved in granule exocytosis. TRX1 and PDIs are secreted from β-cells, and they interact with extracellular domains of transmembrane proteins. PDIs regulate the action of sheddase ADAM17, and TRX1 modifies the activity of integrins and TRPC1/5 channels.