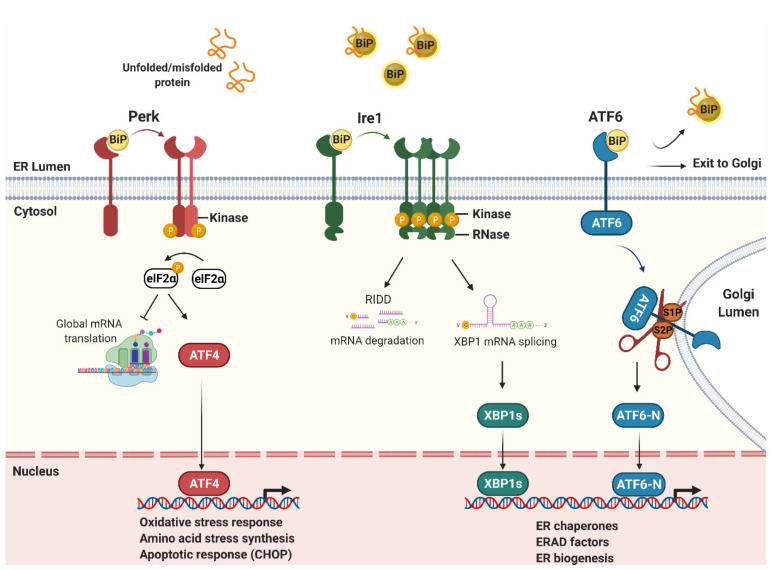
Figure 1.
ER stress and the unfolded protein response (UPR). The UPR is an intracellular signaling cascade activated by misfolded proteins within the ER lumen. Misfolded proteins sequester BiP to release Perk, Ire1 and ATF6 to initiate downstream signaling. Perk is a kinase which phosphorylates the translation initiation factor eIF2α to repress protein synthesis, but selectively increases the translation of ATF4 mRNA via upstream open reading frames (uORFs) in its 5′ untranslated region (UTR). ATF4 is a transcription factor which increases the transcription of the pro-apoptotic factor CHOP. Ire1 is a bi-functional kinase/endoribonuclease (RNase) which splices XBP1 mRNA in the cytoplasm to generate the active transcription factor, XBP1s. Ire1 also cleaves other ER-targeted mRNAs which are then degraded, a process called regulated Ire1-dependent degradation (RIDD). ATF6 is cleaved by site-1 (S1P) and site-2 (S2P) proteases in the Golgi to release its N-terminal transcription factor domain (ATF6-N) that translocates into the nucleus. The single or combined action of XBP1s and ATF6-N up-regulates the transcription of many ER stress-responsive genes to increase ER protein-folding and secretory capacity and to remove misfolded proteins via ER-associated degradation (ERAD).