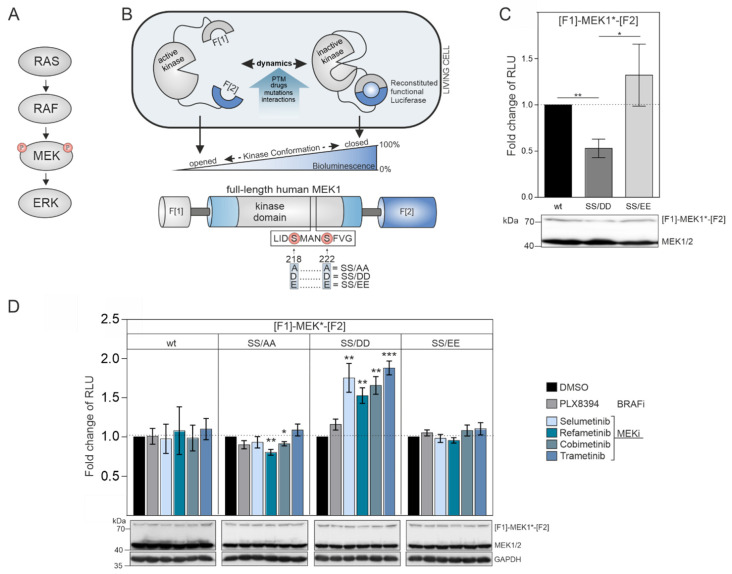
Figure 1.
MEK1 KinCon reporter dynamics: (A) Simplified illustration of RAS-activated signal transmission within the RAS–RAF–MEK signaling pathway. (B) Schematic depiction of the MEK1 KinCon reporter principle indicating fragments 1 and 2 (= F[1]/F[2]) of the Rluc-based PCA (top). Post-translational modifications (PTM), small-molecule binding, kinase domain mutations or protein–protein interactions may convert the MEK1 KinCon reporter into different kinase conformation states which are displayed by alterations of PCA-emitted bioluminescence signals. Domain organization of the MEK1 KinCon reporter iis shown. Activating phosphorylation sites are indicated. Their respective mutations to alanine (SS/AA), aspartic acid (SS/DD) and glutamic acid (SS/EE) are highlighted. (C) Effect of the phospho-mimetic site mutations S218D/S222D and S218E/S222E on MEK1 KinCon dynamics. Bars represent obtained bioluminescence signals in relative light units (RLU) relative to the signals of the wild-type MEK1 KinCon (± SEM from n = 6 independent experiments; normalized on KinCon reporter expression levels, (HEK293T)). (D) Dynamics of indicated MEK1 KinCon reporter signals upon exposure to four MEKi (1 µM), the RAFi PLX8394 (1 µM) or DMSO for 1 h. Bars represent the fold change of RLU relative to the DMSO control of the respective reporter entity (± SEM from at least n = 6 independent experiments, HEK293T). Student’s t-test was used to evaluate statistical significance. Confidence level is indicated by asterisk as: * p < 0.05, ** p < 0.01, *** p < 0.001.