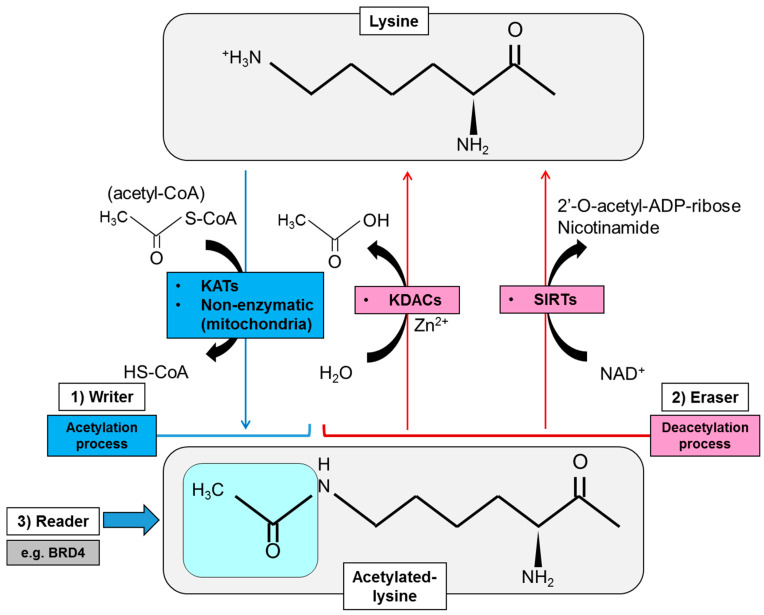
Figure 1.
Regulatory mode of protein lysine acetylation by modification enzymes including writers, erasers and readers of lysine acetylation. Writers of protein acetylation (KATs) transfer the acetyl group from an intermediary metabolite acetyl-CoA to the epsilon NH3+ side chain of lysines of the targeted protein. Acetylation in mitochondrial protein can also be processed non-enzymatically. Acetylation eraser (deacetylase) activity is mediated by Zn2+-dependent KDACs [class I, II and IV histone deacetylases (HDACs)], and class III HDAC or sirtuins (SIRTs) depending on NAD+. The acetylation marks on lysine residues of the histone protein are read by small protein modules called the bromodomain and extra-terminal (BET) proteins including BRD4. ADP, adenosine diphosphate; BRD4, bromodomain-containing protein 4; KAT, lysine acetyltransferase; KDAC, lysine deacetylase; NAD, nicotinamide adenine dinucleotide; SIRT, sirtuin.