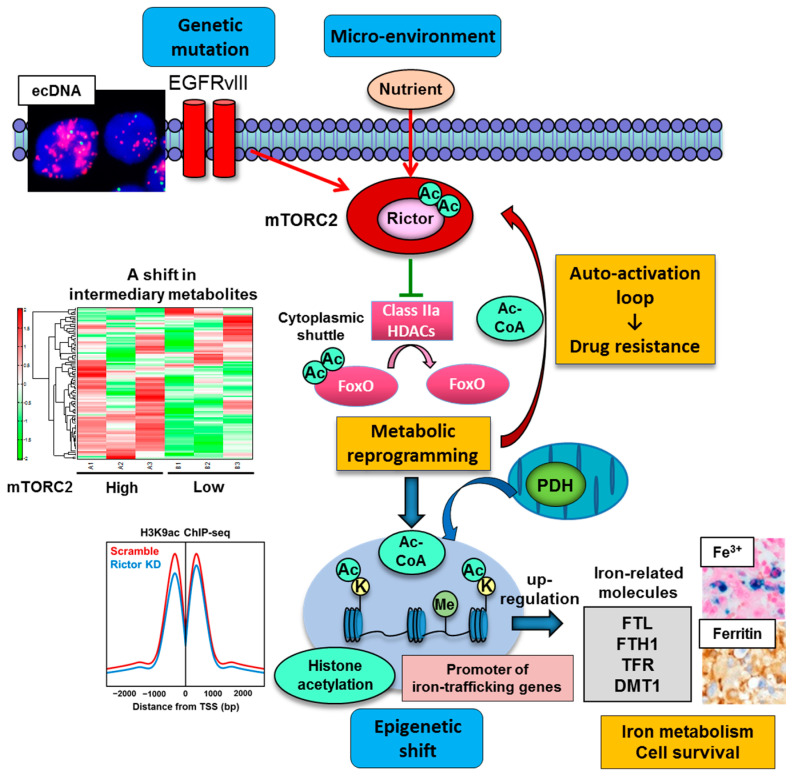
Figure 3.
mTORC2 as a strong acetylation driver in cancer. Genetic mutation including extrachromosomal DNA (ecDNA)-dependent EGFRvIII (epidermal growth factor receptor variant III) overexpression and nutrient in the microenvironment promote mTORC2 activity which facilitates protein acetylation including cytoplasmic protein (FoxO and Rictor) and nuclear histone protein. mTORC2-dependent protein acetylation eventually contributes to c-Myc-dependent metabolic reprogramming of glycolysis, drug resistance to molecular targeting therapies, and epigenetic shift in cancer cells. Of interest, the expression of iron-related genes (FLT, FTH1, TFR, DMT1) is epigenetically promoted by mTORC2-dependent histone acetylation at their promoters, driving iron metabolism and cell survival in cancer. The findings suggest that protein acetylation driven by mTORC2 is a key player to integrate genetics, epigenetics and environment in cancer. Ac, acetyl group; Ac-CoA, acetyl-CoA; bp, base pair; ChIP-seq, chromatin immunoprecipitation sequencing; DMT1, divalent metal transporter 1; FLT, ferritin light chain; FTH1, ferritin heavy chain; FoxO, forkhead box O; HDAC, histone deacetylase; K, lysine residue; KD, knockdown; Me, methyl group; mTORC2, mechanistic target of rapamycin complex 2; PDH, pyruvate dehydrogenase; TFR, transferrin receptor; TSS, transcription start site.