Abstract
Misuse of antibiotics has recently been considered a global issue because of its harmful effects on human health. Since conventional methods have numerous limitations, it is necessary to develop fast, simple, sensitive, and reproducible methods for the detection of antibiotics. Among numerous recently developed methods, aptasensors are fascinating because of their good specificity, sensitivity and selectivity. These kinds of biosensors combining aptamer with colorimetric applications of gold nanoparticles to recognize small molecules are becoming more popular owing to their advantageous features, for example, low cost, ease of use, on-site analysis ability using naked eye and no prerequisite for modern equipment. In this review, we have highlighted the recent advances and working principle of gold nanoparticles based colorimetric aptasensors as promising methods for antibiotics detection in different food and environmental samples (2011–2020). Furthermore, possible advantages and disadvantages have also been summarized for these methods. Finally, the recent challenges, outlook, and promising future perspectives for developing novel aptasensors are also considered.
Keywords: antibiotics detection, colorimetric aptasensors, aptamer-based sensors, antibiotic residue pollution, AuNPs-based aptasensors, aptasensors for antibiotics, colorimetric biosensors
1. Background and Introduction
Antibiotics, also known as antibacterial agents, may have natural or synthetic origin, e.g., microorganisms or numerous chemicals respectively. They are used in the prevention and treatment of bacterial infections [1]. Not only are they involved in saving patients’ lives, but they also play a key role in surgery and medicine-based advances [2]. Despite their overuse warnings, they are commonly overprescribed in many countries [3]. Using an overdose drives resistant bacterial evolution. In both developing and developed countries, they are widely used in livestock as growth supplements [4]. Subsequently, they are consumed by humans through ingesting those animal-derived foodstuffs [3]. Resistant bacterial species are also transmitted through the food chain to humans, causing severe infections leading to serious health conditions. The use of antibiotics in agricultural practices also have influences on environmental microbiome [4]. As a result, increased mortality and morbidity due to infections and compromised immune-system becomes virulent [3]. Thus, it is crucial to develop useful methods to cope with the long-term life-threatening effects caused by these antibiotic residues in the environment and food for their effectual monitoring [5,6].
At present, a variety of methods such as high-performance liquid chromatography (HPLC) [5,7,8,9,10], liquid chromatography-mass spectrometry [5,11,12,13,14], chemiluminescence (CL), immunoassays, electrochemical and capillary electrophoresis [5,15], gas chromatography-mass spectrometry [16], etc. are available for the specific detection of a variety of antibiotics. Liquid chromatography in combination with mass spectrometry (LC-MS) guarantees high resolution, target identification, selectivity, repeatability, sensitivity and application versatility [17]. The most common, currently-used approaches to confirm antibiotic residue presence are LC-MS, ELISA, and biosensor-based methods. All these methods are associated with some notable advantages (e.g., practicality and sensitivity, etc.) and disadvantages (e.g., unsatisfactory cost-effectiveness and slowness, etc.) [18] which do not impinge on the validity of the above reported methods.
2. Biosensors
Biosensors circumvent the above-mentioned limitations to ensure a fast in situ analysis [19]. These kinds of detection techniques exhibit high sensitivity, specificity, excellent performance and are easy-to-use or inexpensive [20]. Biosensors are desirable for rapid or accurate detection of environmental pollutants, materials, biological and chemical stuff [21]. A biosensor is a device involving two main parts in close proximity: a bio-recognition element (BRE) and a signal transduction element (STE) (Figure 1A). BRE comprises two affinity-pairing partners, e.g., receptor/specific ligand, enzyme/substrate and antibody/antigen, etc. One of the partners is usually immobilized [18]. BRE is an essential component of a biosensor. Most common BREs include aptamers, enzymes, molecularly imprinted polymers, antibodies, and many more. These BREs are known to have high specificity and selectivity for their specific targets [20]. The STE is used to detect any interactions between the two affinity-pairing partners and thus converts the biological signals into useful electrical signals [18]. Finally, detection system monitors these signals for further analysis [20]. Biosensors can be grouped or classified based on their STEs and BREs [18]. Based on the choice of BREs, biosensors are classified as aptasensors, MIP-based biosensors, and immune-sensors [20] (Figure 1A).
Figure 1.
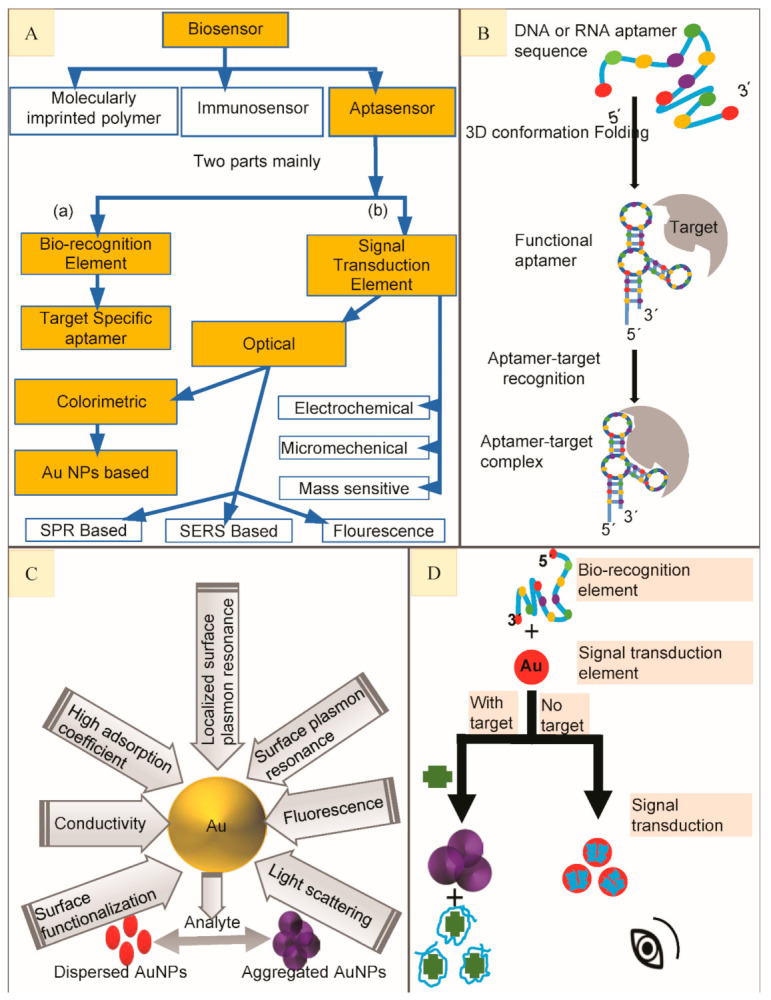
(A) Scheme to show main types of biosensors to highlight aptasensor, classification of aptasensors based on its major components to highlight Gold Nanoparticles (AuNPs) based colorimetric aptasensors. (B) Generalized depiction of an aptamer library folding to form secondary structure in order to bind with high affinity target molecule to finally make aptamer-target complex. (C). Properties of Gold nanoparticles and calorimetry. (D) A generalized illustration on working principle of colorimetric aptasensor when bio-recognition element (BRE) combines with signal transduction element (STE) to make a colorimetric aptasensor to detect small molecular targets.
Biosensors still have their own drawbacks mostly around sterilization, cost and stability. Main disadvantage of these sensors is the uncertainty of biological recognition/sensing element which may affect the overall sensing mechanism by environmental factors (temperature, ionic strength and pH), duration of use and type of molecules. They may have problems associated with transduction element size [18].
3. Aptasensors and Aptamers
A kind of biosensor using aptamers as BRE is known as aptasensor [20]. Aptamers are synthetic single-stranded oligonucleotide sequences (RNA or DNA) with high specificity and affinity to bind a variety of target classes including proteins, peptides, drugs, small molecules, whole cells, inorganic and organic molecules, etc. [22]. Aptamers are especially screened via an in vitro selection process named SELEX (systematic evolution of ligands by exponential enrichment) [23], in situ SELEX [24], in-silico/computational approaches [25] and some bioinformatic methods to combine both the in silico and in vitro approaches, etc., to identify the best fit for each target analyte [26]. They have several other striking features such as high stability, greater affinity, low molecular weight, easy and reproducible preparation [27]. They undergo a folding process to make a specific three-dimensional structural rearrangement in order to bind with their particular target (Figure 1B). Three-dimensional structure depends on aptamer sequence, primary structure, pH, temperature and buffer composition of the sensor [28]. Target-aptamer interactions mainly depend on the nature of its binding analyte, three-dimensional structure of the aptamer and distribution of their charges [29]. Interaction types between aptamer target complex involve electrostatic interactions, van der Waals forces, shape complementarity, stacking of flat moieties and hydrogen bonding so that their dissociation constants (Kd) values range from pico- to nanomolar levels [30].
Aptamers can also be referred to as “chemical antibodies” since they mimic antibodies in their applications and characters. Although aptamers are advantageous over antibodies in many aspects especially because of their high affinity, strong specificity, low immunogenicity, targeting a broad range of analytes, cost effectiveness and short preparation cycle [31] they are not as commonly used as antibodies, however developing rapidly [32]. Performance of different aptasensors can be compared keeping in view some important characteristics or parameters of their constituent aptamers such as affinity, specificity, selectivity, limit of detection (LOD), and stability, etc.
4. Important Parameters to Measure the Efficiency of Aptasensors
The word affinity stands for the strength of interactions existing between the target and its specific aptamer (of an aptasensor), is measured by approaching the association or binding constant (Ka) that is inversely proportional to the dissociation constant (Kd). Aptamer sequences with lowest Kd values have been considered to show strong target interactions. Hence, high-affinity aptamers may bind to even small quantities of target molecules present in the sample (make them more sensitive) known to have a low limit of detection [33]. The selective aptamer binding to only its desired analyte without any cross-reactivity to some non-targeted analytes from an entire sample mixture is termed as the specificity which is a crucial parameter to diminish false-positive results [34]. However, aptamers could not be absolutely specific (in an aptasensor), they can be selective to bind their desired target analytes in the best way and many folds lesser than non-targeted analytes. Because attaining 100% specificity might not be promising, efforts are being made to produce aptamers as selective as possible [33]. The LOD is referred to be the minimum detectable quantity of an analyte in comparison to a blank sample (in the absence of that particular analyte) [35]. The key objective in constructing an efficient biosensor is to approach the highest sensitivity with a smaller LOD than the minimum residual level [36]. The stability of an aptasensor is the capacity to conserve its efficient performance under prevalent situations for a time being. It can be verified by storing the biosensor in a set of specified conditions for a couple of weeks and then relating the analytical efficiency before and after storage [19].
5. Classification of Aptasensors
In addition to the built-in biosensor advantages, apta-sensors take another advantage of reusability over the conventional antibodies [37]. Aptamer based biosensors depending on STE have been classified as optical, mass-sensitive, micromechanical and electrochemical sensors [38]. Optical assays have been extensively developed for their simple operation, quick response and high sensitivity. Combining aptamer as BRE with optical bioassays as STE, offer unique advantages to optical aptasensors [39,40]. This kind of aptasensors can be grouped on the basis of their light absorption properties (when they are exposed to different analytes) and luminescence changes [41]. Optical aptasensors are mainly classified into four types including colorimetry, fluorescence, surface-enhanced Raman scattering (SERS) and surface plasmon resonance (SPR) [40] (Figure 1A). In this review, we will focus on AuNPs-based colorimetric aptasensors only.
6. Gold Nanoparticles (AuNPs) Based Colorimetric Aptasensors
Nanomaterials are widely used for their excellent sensing application in biosensing with favorably lowered detection limits and higher sensitives. Almost all nanomaterials take the advantage of high specific surface allowing the immobilization of an enhanced amount of bio-recognition element (BRE) to substitute the classic transduction methods. Covalent linkage of nanomaterials to biomolecules is attributed towards the lowering of unspecific physisorption, stability of the system and the reproducibility of the surface functionalization. A variety of nanomaterials, each with their own unique properties are known to enhance biosensor performance [42].
The combination of aptamers with AuNPs has been widely used to develop colorimetric aptasensors for optical recognition of antibiotics [43]. AuNPs have attracted greater attention owing to their light-scattering properties, strong optical absorption, unique chemical, biological, electronic properties, low or no toxicity and fluorescence quenching, etc. [44,45] (Figure 1C). Their unique optical properties are the result of mutual electronic oscillations at their surface which can be controlled by regulating their sharpness, composition and size (diameter) especially. Their diameter can be easily controlled by various experimental parameters to shape-up the desired optical features [46]. Fabricating colorimetric aptasensors by the use of AuNPs is gradually getting popularity for their simple operation and easy preparation [47]. The STE is an exceedingly essential part of an aptasensor while designing colorimetric assays with AuNPs because of their special optical properties, e.g., high extinction coefficient and surface plasmon resonance (SPR) [48]. The aptamers are adsorbed on AuNPs surface (in the absence of their high-affinity target) to stabilize them, thus preventing their salt-induced aggregation because of SPR connection among adjoining particles [49]. Contrarily, on target addition to the aptasensor, aptamer binds to their high affinity target, causing aptamer desorption from AuNPs surface, thus aggregating the AuNPs leading to a color change from bright red to blue or purple (Figure 1D) most commonly (the absorbance peak shifts from 520 to 650 nm) because their unique localized surface plasmon resonance (LSPR) is changed. These absorbance peak shifts could be identified by collecting them on reflectance signals [50] and then be quantified by using a UV–Vis spectrophotometer [49]. As their color changes are very obvious, it can be easily detected by the naked eye. This kind of aptasensors is more appropriate for their in-situ applications where they can greatly facilitate simple and rapid routine inspections [51]. In this review, we have summarized the performance dependent analysis of almost all of the colorimetric gold nanoparticles-based aptasensors reported till now with a special focus on the detection of antibiotics. Colorimetric aptasensors reported for different antibiotics have been outlined individually with respect to the particular group they belong.
AuNPs diameter is the most important factor in all the reported methods with most of the protocols using the particle size between 10 and 27 nm diameter (most of the methods used 13 or 15 nm) of AuNPs, prepared by classical citrate reduction of HAucl4 or their modifications for example the method used by Huang et al. [52] to prepare AuNPs. Antibiotics have been classified into seven groups on the bases of their functions and molecular structure, including β-lactams (BLCs), aminoglycosides (AGSs), anthracyclines (ACs), chloramphenicol (CAP), fluoroquinolones (FQs), tetracycline (TC) and sulfonamides (SAs) Table 1.
Table 1.
Classification of seven antibiotic classes (basic structure highlighted in green), and their different derivatives (alterations from green to black molecular structures).
| Antibiotic Class | Antibiotic | Molecular Structures |
|---|---|---|
| β-Lactams (BLCs) |
Ampicillin (AMP) |
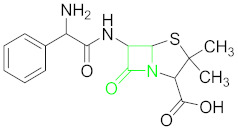
|
| Aminoglycosides (AMGs) |
Kanamycin (KAN) |
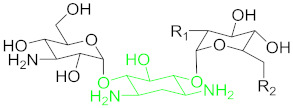
|
| Tobramycin (TOB) |
|
|
| Streptomycin (STR) |
|
|
| Neomycin B (NB) |
|
|
| Anthracyclines (ACs) |
Daunomycin (DNR) |
|
| Chloramphenicol | Chloramphenicol (CAP) |
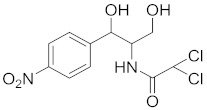
|
| Fluoroquinolones (FQs) |
Ciprofloxacin (CIP) |
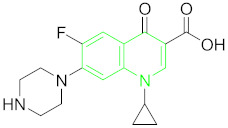
|
| Tetracyclines (TCs) |
Oxytetracyclines (OTC) |
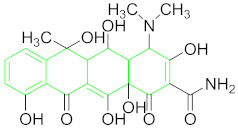
|
| Tetracycline (TET) |
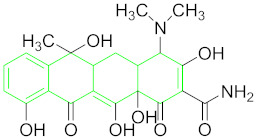
|
|
| Sulfonamides (SAs) |
Sulfadimethoxine (SDM) |
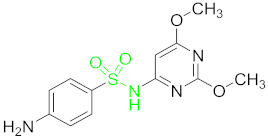
|
6.1. β-Lactams and Their Residue Detection
β-lactams are a class of antibiotics, which includes cephalosporins, penicillin, carbapenem and monobactams, and represents an effective medicine widely used in the treatment of mastitis courses, pulmonary, urinary and septicemia conditions [53]. They also find uses in boosting animal growth [54]. They are characterized by their representative β-lactam ring [19] (Table 1). An over-use of these substances results in their residues to be accumulated in food, surface water, soil, etc., predominantly in animal-derived foods and other dairy products [55,56]. It is extremely important to detect the traces of these contaminants in all kinds of foodstuffs as otherwise they may pose severe health concerns for human beings, ranging from resistant bacterial evolution to allergic reactions in individuals [57] and bacterial resistance related infections [58]. The most important details of the AuNPs based colorimetric aptasensors being discussed for the antibiotics of each group individually, including the aptamer sequences, dissociation constant (KD), the limit of detection (LOD), color change, AuNP particle size, and synthesis protocol are listed in their respective tables for each antibiotic class (Table 2).
Table 2.
Summary of important parameters/results for each method discussed (aptamer sequence, linker and spacers, dissociation constant (Kd), limit of detection (LOD), color change in the presence or absence of target, AuNPs particle diameter and preparation methods for each method, mentioned in the corresponding references (Ref.).
| Target | 5′ Linker and Spacer | Aptamer Sequences 5′→3′ | 3′ Linker and Spacer | KD (nM) |
LOD (nM) |
Color Change | AuNP Particle Size (nm) | AuNP Preparation Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AMP | FAM | AMP17: GCG GGC GGT TGT ATA GCG G AMP18: TTA GTT GGG GTT CAG TTG G AMP4: CAC GGC ATG GTG GGC GTC GTG |
biotin | 13.4 9.8 9.4 |
14.3 (dw) 28.6 (m) |
Red-purple | 13 | classical citrate reduction method | [59] |
| AMP | (SH)–(CH2)6 | -GCGGGCGGTTGTATAGCGG GCGG GCGGTTGTATAGCGG- |
(T)15–(A)12 | 0.10 0.49 |
10 50 |
Red-purple Red-purple |
10 | Smith method | [60] |
| KAN | - | TGG GGG TTG AGG CTA AGC CGA | - | 8.38 | 1.49 | Ruby red to purplish-blue or colorless |
- | classical citrate reduction method | [61] |
| KAN TOB |
- | TGGGGGTTGAGGCTAAGCCGA | - | KAN = 78.8 KAN B = 84.5 TOB = 103 |
25 | Red-purple | 13 | classical citrate reduction | [62] |
| KAN SDM |
- | SDM; GAGGGCAACGAGTGTTTATAGA KAN: TGGGGGTTGAGGCTAAGCCGA- |
- | - | - | Red-purple/blue | 13 | Classical citrate reduction | [63] |
| KAN | - | (KBA) 3-1; CGG AAG CGC GCC ACC CCA TCG GCG GGG GCG AAG CTT GCG Ky2; TGG GGG TTG AGG CTA AGC CGA |
- | - | 3.35 26.8 |
wine red to purple blue | 14, 21, 27 | citrate reduction of HAuCl4 | [64] |
| KAN | Apt; HS–(CH2)6 DNA1; SH–(CH2)6 DNA2; biotin |
Apt; TGG GGG TTG AGG CTA AGC CGA DNA 1: TCA GTC GGC TTA GCC GTC CAA CGT CAG ATC C DNA2: CCG ATG GAT CTG ACG T |
apt: biotin | - | 0.0778 | Red- colorless or faint red | 13 | citrate reduction of HAuCl4 | [65] |
| KAN | ssDNA1; HS–(CH2)6- | KSA; TGGGGGTTGAGGCTAAGCCGA ssDNA1; CGGTCGGCTTA ssDNA2; AACCCCCATCT |
ssDNA2; (CH2)3–SH | - | 01 | Red-purple | 13 | citrate reduction of HAuCl4 | [66] |
| KAN | KMC apt; NH2 | KMC apt; TGGGGGTTGAGGCTAAGCCGA cDNA; TCGG CTTAGCCTCAACCCCCA |
- | - | 2.5 | Pink to white | 15–20 | citrate reduction method | [67] |
| KAN | - | TGG GGG TTG AGG CTA AGC CGA | (T)15–(A)12 | - | 0.05 | Red to blue | 15 | citrate reduction method | [68] |
| TOB | - | GGG ACT TGG TTT AGG TAA TGA GTC CC | - | - | 23.3 | red-purple-blue | 13 | classical citrate reduction | [49] |
| TOB | - | - | - | - | 37.9 | red to purple | - | - | [69] |
| STR |
- | I: GGG GTC TGG TGT TCT GCT TTG TTC TGT CGG GTC GT II: TGA AGG GTC GAC TCT AGA GGC AGG TGT TCC TCA GG III: AGC TTG GGT GGG GCC ACG TAG AGG TAT AGC TTG TT IV: TGT GTG TTC GGT GCT GTC GGG TTG TTT CTT GGT TT |
- | I: 199.1 II: 221.3 III: 272.0 IV: 340.6 |
200 | red to purple | 13 | citrate reduction of HAuCl4 | [70] |
| STR | I: FAM II: FAM III: FAM |
I: CCC GTT TAA AGT AGT TGA GAG TAT TCC GTT TCT TTG TGT C II: GTG CGT TAT AAA CTA GTT TTG ATT CAA TGT TGG GTG TGG G III: GGG CCT GTT TTG CCT TCA CGT TCT CTT CCT TGC CGT TCT G |
I: biotin II: biotin III: biotin |
I: 6.07 II: 8.56 III: 13.14 |
25 nm/L | red to purple | 15 | citrate reduction of HAuCl4 | [71] |
| STR | SH–(CH2)6- | TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G | - | - | 0.0017 (b) 0.001 |
colorless to blue | 19.73 | Au NPs–PV | [72] |
| STR | - | TAGGGAATTCGTCGACGGATCCGGGGTCTGGTGTTCTGCTTTGTTCTGTCGGGTCGTCTGCAGGTCGACGCATGCGCCG | - | 19.1 | 86 | red-grayish green | 13 | citrate reduction of HAuCl4 | [73] |
| STR | - | STR apt: TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G CS; C GGC GCA TGC GTC GAC CTG CAG ACG ACC CGA CAG AAC AAA GCA GAA CAC CAG ACC CCG GAT CCG TCG ACG AAT TCC CTA |
- | - | 73.1 (b) 102.4 (b) 108.7 (m) |
red to blue | 15 | citrate reduction of HAuCl4 | [74] |
| NB | - | AP-W; GGACUGGGCGAGAAGUUUAGUCC Ap-M18; GGACUAAACGAGAAGUUUAGUCC Ap-M20; GGACUAAACGAGAAGCCCAGUCC |
- | - | 470 27 360 |
pink red to Blue | 13 | citrate reduction of HAuCl4 | [75] |
| DNR | - | GGGAATTCGAGCTCGGTACCATCTGTGTAAAAGGGGTGGGGGTGGGTACGTCTAGCTGCAGGCATGCAAGCTTGG | - | - | 17.1 | red to purple | 13 | reduction of HAuCl4 | [76] |
| CAP | AuNPs DNA; Biotin | Apt; ACTTCAGTGAGTTGTCCCACGGTCGGCGAGTCGGTGGTAG AuNPs binding DNA; ACTTTCCATTCCTTTTAC |
Apt; Biotin AuNPs DNA; Thiol |
- | 0.451 (b) 0.697 (m) 0.601 | bright red to faint red | 14 | reduction of HAuCl4 | [77] |
| CAP | apt: SH–(CH2)6 cDNA: SH–(CH2)6 |
apt: ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G cDNA: CTA CCA CCG ACT CGCG CGA CCG TGG GAC AAC TCA CTG AAG T |
- | - | 0.00093 (b) (0.3 × 10−9 g/L) | colorless to light blue |
20 | slight modified Frens method | [78] |
| CAP | apt: SH–(CH2)6 cDNA: SH–(CH2)6 |
apt: ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G cDNA: TTT TCT ACC ACC GAC TCG C |
- | - | 0.02 ng/mL | colorless to light blue | 20 | slight modified Frens method | [79] |
| CAP | Apt; SH–(CH2)6 cDNA: SH–(CH2)6 |
Apt; ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTAG cDNA: CTA CCA CCG ACT CGC CGA CCG TGG 142 GAC AAC TCA CTG AAGT |
- | - | 0.046 (b) (0.015 × 10−6 g/L) | colorless to light blue | 16 | slight modified Frens method | [78] |
| CAP | Apt;Biotin DNAzyme; (SH–(CH2)6– cDNA; (SH)–(CH2)6 |
Apt; ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G DNAzyme; AAAAAA GGG TAG GGC GGG TTG GG cDNA; AAA AAA AAA AAA AAA AAA AAA AAA AAA AAA CTA CCA CCG ACT CGC C |
- | - | 0.13 pg/mL | red to blue | 13 | reduction of HAuCl4 | [80] |
| CAP | - | ACTTCAGTGAGTTGTCCCACGGTCGGCGAGTCGGTGGTAG | - | - | 7.65 5.88 |
red to blue | 15 | Huang et al. | [51] |
| CAP TET |
- | Multi-apt; ACTTCAGTGAGTTGTCCCACGGTCGGCGAGTCGGTGGTAGCGGTGGTG | - | - | CAP.7.0 TET.32.9 |
(CAP) wine red to purple (TET) wine red to blue |
15 | Huang et al. | [81] |
| CAP | - | Apt; ACT TCA GTG AGT TGT CCC ACGGTC GGC GAG TCG GTG GTAG LP; CTGAAGTTCTACCAC |
- | - | 0.03 | pink red to blue | 14 | Classical reduction of HAuCl4 | [82] |
| FQs CIP |
CS2; Thiol | Apt; ATACCAGCTTATTCAATTGCAGGGTATCTGAGGCTGATCTACAATGTCGTGGGGCATTTATTGGCGTTGATACGTACAATCGTAATC AGTTAG CS1; TTGAATAAGCTGGTATAAACC CS2; AAACCACCTCCGAATCCCAAGCCACC GCCGCTAACTGATTACGATTGT |
CS1; Thiol | - | 1.2 (CIP) 1.3 (w) 2.6 (bs) 3.2 (m) |
yellow to colorless | 12 | classic citrate reduction | [83] |
| OTC | - | CGTACGGAATTCGCTAGCGGGCGGGGGTGCTGGGGGAATGGAGTGCTGCGTGCTGCGGGT CCGAGCTCCACGTG- | - | - | 25 | red to purple | 13 | citrate reduction of HAuCl4 | [84] |
| OTC | - | CGTACGGAATTCGCTAGCACGTTGACGCTGGTGCCCGGTTGTGGTGCGAGTGTTGTGTGGATCCGAGCTCCACGTG | - | - | 01 | red to purple | 13 | citrate reduction of HAuCl4 | [50] |
| OTC and KAN |
- | OTC apt; CGTACGGAATTCGCTAGCGGGCGGGGGTGTGGGGGAATGGAGTGCTGCGTGCTGCGGGGT CCGAGCTCCACGTG KAN apt; TGG GGG TTG AGG CTA AGC CGA |
- | - | 1 ag mL−1 | colorless to blue or yellow | 10 | citrate reduction of HAuCl4 | [85] |
| OTC | - | CGA CGC ACA GTC GCT GGT GCG TAC CTG GTT GCC GTT GTG T | - | - | 10 (w) 20 (m) |
red to blue | 13 | Classical citrate reduction | [5] |
| TET | - | CGTACGGAATTCGCTAGCCCCCCGGCAGGCCACGGCTTGGGTTGGTCCCACTGCGCGTGGATCCGAGCTCCACGTG | - | - | 122 | purple to red | 13 | citrate reduction of HAuCl4 | [86] |
| TET | - | CGTACGGAATTCGCTAGCCCCCCGGCAGGCCACGGCTTGGGTTGGTCCCACTGCGCGTGGATCCGAGCT CCACGTG | - | - | 45.8 | red to purple | 15 | citrate reduction of HAuCl4 | [86] |
| TET | - | Apt; CTCTCTCGGTGGTGTCTCTC Signal Transduction Probe; GAGAGAGAGAGAGA |
- | - | 266 pM | red to blue | 15 | classical citrate reduction of HAuCl4 | [87] |
| TET | - | CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCGTGG ATC CGA GCT CCA CGT G | - | - | 0.039 µg/mL | red to blue | 13 | - | [88] |
| TET | SH–(CH2)6 | CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT G | - | - | 0.002 ng/mL, | colorless to blue | 18 | classical citrate reduction of HAuCl4 | [89] |
| SDM | - | SDM apt; GAGGGCAACGAGTGTTTATAGA KAN apt; TGGGGGTTGAGGCTAAGCCGA |
- | - | 50 ng/m | red to blue | 13 | Mayer’s method | [90] |
| SDM | - | GAGGGCAACGAGTGTTTATAGA | - | - | 10 ng/mL | red to purplish-blue | 13 | citrate reduction method | [91] |
| SDM | - | SDM apt; GGC AAC GAG TGT TTA cDNA; TAA ACA CTC GTT GCC |
- | - | 3.41 ng mL−1 (w) 4.41 ng g−1 (f) |
red to blue/purple | 13 | citrate reduction method | [92] |
Ampicillin (AMP), Kanamycin (KAN), Tobramycin (TOB), Streptomycin (STR), Neomycin B (NB), Daunomycin (DNR), Chloramphenicol (CAP), Ciprofloxacin (CIP), Oxytetracycline (OTC), Tetracycline (TET), Sulfadimethoxine (SDM), apt = aptamer, cDNA = complementary DNA, FAM = fluorescein amidite, m = milk, dw = distilled water, w = water, f = fish, bs = blood serum, and b = blood.
A penicillin-like antibiotic, ampicillin (AMP) (Table 1) is usually used to treat different bacterial infections, e.g., bronchitis, pneumonia, ear, urinary tract, lung and skin infections [93]. AMP is extensively used in agriculture and medicine for the treatment of bacterial infections and is a member of penam class of beta-lactams. An overdose of AMP could cause undesirable accumulation of its residues in foodstuffs and may lead to serious complications, such as breathing difficulties, seizures, and allergic reactions in humans [94,95]. The first aptamer-based sensor for AMP dual detection (fluorescence–colorimetric) was reported by Song et al. based on AuNPs and ssDNA (selected by magnetic bead-based SELEX). AuNPs acted as a signal probe in developing color for colorimetric assay and as a quencher using fluorescence in a dual-detection system at the same time [59].
In another method, Shayesteh and Ghavami designed a comparative study to investigate the adsorption efficiency of two different aptamers (T-Apt and polyA Apt) on the surface of AuNPs for AMP detection. Their findings suggested that polyA Apt could be a better substitute for thiolated-Apt in the construction of aptasensor to detect a number of analytes including AMP [60].
6.2. Aminoglycosides and Their Residue Detection
Aminoglycosides represents an antibiotics class, including kanamycin, tobramycin, streptomycin and neomycin, etc., derived from several Streptomyces species [96]. All of these members share a common hexose ring in their structure, normally streptidine for streptomycin molecule or 2-deoxystreptamine and numerous glycosidically linked amino sugars [97]. Aminoglycosides are known to have broad-spectrum activity, they are mostly used as veterinary drugs to cure infections by aerobic Gram-negative bacteria and many other bacteria. The detection of traces of these drugs in nutrition is considered a big health hazard for the consumers [98].
6.2.1. Kanamycin (KAN)
Kanamycin (Table 1) is an important subclass of aminoglycosides being used widely for the treatment of serious bacterial infections (Gram-negative and Gram-positive) by protein synthesis interference. It shows a narrow range of therapeutic index. An overuse of this medicine in animal-derived nutrients may have serious antibiotic resistance, nephrotoxicity, and ototoxicity in humans [40]. Thus, in order to eradicate these harmful effects, selective, accurate, sensitive and simple detection methods for kanamycin monitoring in animal-derived foodstuff and serum are direly needed [99]. Since selective or sensitive KAN residues detection methods for clinical diagnosis and food safety are highly demanding, more reports have been found that are related to KAN aptasensors than other antibiotics.
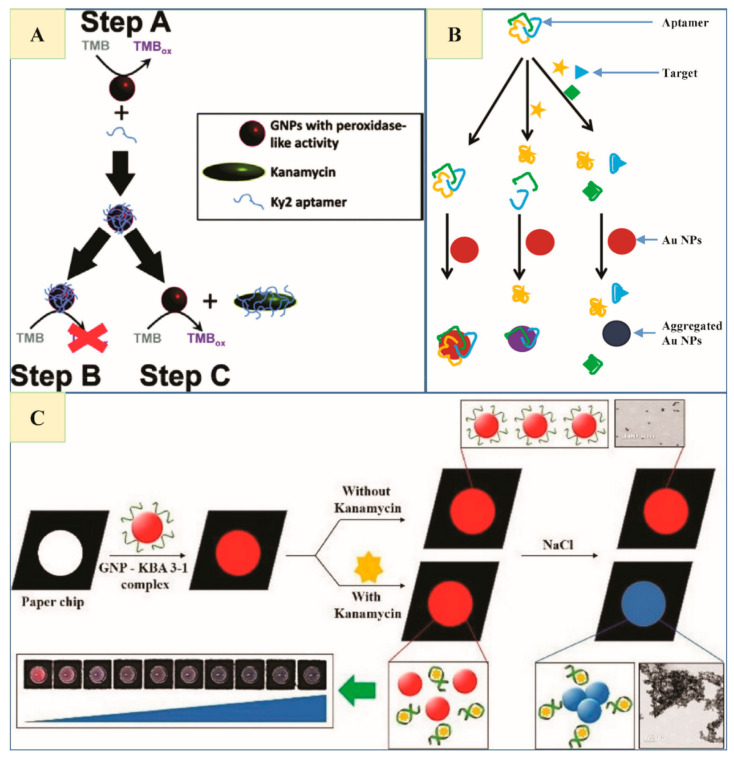
A highly sensitive and ultrafast ‘turn-on/turn-off’ method was approached by Sharma et al. that combines Ky2 (ssDNA) aptamer with tyrosine-reduced AuNPs as a peroxidase nanozyme to detect KAN. Nanozyme activity of AuNPs eliminates the necessity for NaCl induced aggregation bringing about faster readouts and improved sensitivity. Overall, a one-step, straightforward, and simple method was developed to take place in 3–8 min [61]. The working principle is shown in Figure 2A.
Figure 2.
(A) Schematic depiction of the ‘turn-on/turn-off’ nanozyme catalytic activity of aptamer-functionalized AuNPs for Kanamycin (KAN) detection (Step A) showing intrinsic peroxidase activity of AuNPs when they ‘turn-off’ after functionalization with Ky2 in the absence of KAN (Step B), and ‘turn-on’ again in the presence of KAN (Step C) (Reproduced with permission from [61] © 2014 Royal Society of Chemistry). (B) A schematic illustration of multiplex antibiotic detection using a colorimetric aptasensor based on a simple working principle (Reproduced with permission from [63] © 2014 Plos ne). (C) A schematic demonstration of a paper chip-based AuNPs colorimetric aptasensor for KAN detection (Reproduced with permission from [64] © 2017 Elsevier).
Song et al. reported a high-affinity ssDNA aptamer for KAN by SELEX (using affinity chromatography). Thus, they constructed a biosensor based on salt induced aggregation of unmodified AuNPs upon aptamer dissociation in the presence of KAN which binds to the loop region, especially the GG region, of the Ky2 aptamer. This low-cost sensor is used in food safety and pharmaceutical preparations [62].
A multiplex colorimetric aptasensor by adsorbing more aptamers (from more than one class, mixed at a particular ratio) on AuNPs surface was designed by Niu et al. to detect more than one antibiotic simultaneously (e.g., KAN, SDM and ADE). In the presence of any one or more targets, the corresponding aptamer undergoes conformational changes to dissociate from AuNP surface- to make aptamer-target complex. AuNPs undergoe aggregation, leading to specific color changes [63] (Figure 2B). The multiplex aptamer-based sensor described above has its own limitations; for example, it fails to determine which analytes were detected from a multiplex sample showing a positive result (Table 3). However, in various situations, e.g., veterinary drug traces determination in animal production, a great deal of different samples need screening for more than one analyte simultaneously. Their multiplex aptasensor working principle can be extended successfully for various other analytes detection by changing the specified aptamer sequences for the desired targets.
Table 3.
Summary of advantages and disadvantages of the reported methods to make a comparison within each antibiotic class and with different classes.
| Antibiotic Group/Class | Advantages | Disadvantages |
|---|---|---|
| β-Lactams/(AMP) |
|
- |
|
Aminoglycosides/
(KAN), (TOB), (STR), (NB) |
|
|
|
Anthracyclines/
(DNR) |
|
- |
|
Chloramphenicol/
(CAP) |
|
|
|
Fluoroquinolones
(CIP) |
|
- |
|
Tetracyclines/
(OTC), (TET) |
|
- |
|
Sulfonamides
(SDM) |
|
A label-free, nitrocellulose membrane paper chip-based, simple method to visualize a quick absence/presence analysis of chemical reactions by the naked eye, was reported by Ha et al. However, a quantitative analysis can be performed using color and image analysis software. In the presence of KAN, kanamycin binding aptamer is detached from AuNPs surface (to make aptamer-target complex) to induce AuNPs aggregation and bring about the color changes in solution Figure 2C [64].
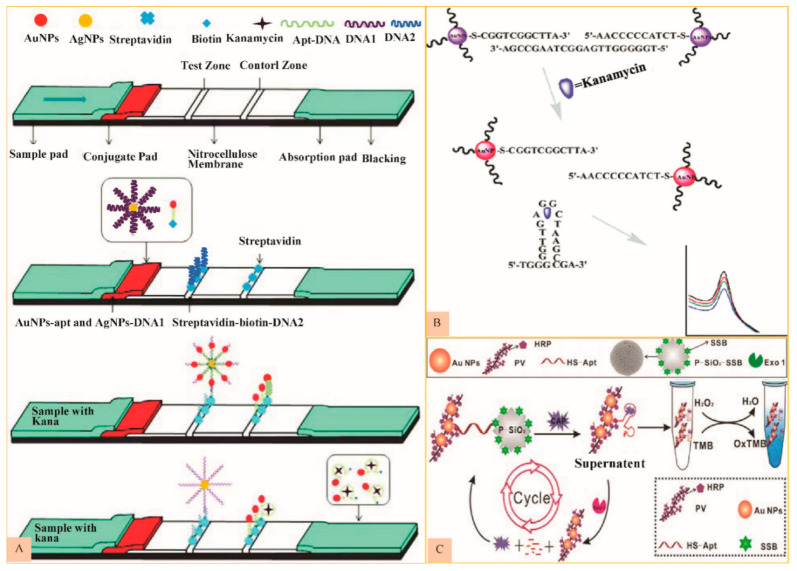
A strip based, lateral flow, on-site KAN detection aptasensor was introduced by Liu et al. in which aptamer modified AuNPs act as a probe (AuNPs-apt) while oligonucleotide modified AgNPs as (AgNP-DNA1) signal amplification element. Another complementary capture probe DNA2 was immobilized on the 3’- terminal of AgNPs-DNA1-apt-AuNP complex on the strip. The working principal of this low cost, highly specific and stable aptasensor is shown in Figure 3A, signifying its potential for in-field KAN testing for food safety applications [65].
Figure 3.
(A) Schematic figure showing system alignment and detection principle of strip aptasensor for KAN (Reproduced with permission from [65] © 2018 Royal Society of Chemistry). (B) Schematic diagram to represent spectrophotometric kanamycin detection (Reproduced with permission from [66] © 2014 Royal Society of Chemistry). (C) Scheme depicting the suggested biosensing of STR dependent on the developed colorimetric aptasensor (Reproduced with permission from [72] © 2017 Elsevier).
Zhou, Zhang et al. developed an AuNPs based spectrophotometric aptasensor for KAN detection in milk. Functionalized AuNPs were aggregated upon the addition of KAN specific aptamer because aptamer forms a hybrid with complimentary strand already attached to AuNPs surface. However, upon KAN addition, it binds competitively to the aptamer, disaggregating the AuNPs and a visible change in absorption spectrum of their solution (Figure 3B) [66].
A novel strip-based, portable and stable colorimetric aptasensor was constructed by Abedalwafa et al. in which a nanofibrous membrane carrier having carboxyl group was fabricated by grafting glutamic acid (GA) into cellulose acetate (CA) (G-CA NFMs). KAM aptamer was then labeled on G-CA NFMs. A complimentary strand (cDNA) (as signal probe) of KAM apt-conjugated AuNPs was hybridized with aptamer on G-CA NFMs. KAM addition can disassemble the signal probe by replacing cDNA to bind with aptamer. The method showed excellent results to detect KAN in milk and drinking water [67].
Shayesteh and Khosroshahi introduced the latest strategy based on AuNPs aggregation controlled by the specific interactions between the cationic polymer (PDDA), KAN presence/absence and anti-kanamycin aptamer. AuNPs are stable with aptamer combined PDDA on their surface. On KAN addition, aptamer dissociated from AuNPs surface to bind KAN. The method is used to detect KAN concentration in serum clinically [68].
6.2.2. Tobramycin (TOB)
Tobramycin (Table 1) is an aminoglycoside antibiotic, with broad-spectrum activity, derived from Streptomyces tenebrarius [97]. TOB is a cheap drug, widely used in animal farms for animal husbandry leading to its residue accumulation in food chain from animal derived food, such eggs, meat and milk, etc. [49]. Because of its adverse effects such as nephrotoxicity and ototoxicity, it shows a narrow therapeutic range in blood serum same as other aminoglycosides. Monitoring of blood serum levels before therapy is of critical importance for these reasons [97].
Han et al. screened ssDNA aptamers through magnetic bead-based SELEX which they truncated and used to develop AuNP-based photometric aptasensor. Theoretical modeling exhibited that nucleotides 14–18 and 26–29 play a considerable role in aptamer and tobramycin interactions. A truncated 34 nucleotides aptamer was finally optimized in an aptasensor which has promising results in TOB detection in honey samples [69].
TOB detection was designed by Ma et al. based on ssDNA and unmodified AuNPs. The high specificity and sensitivity aptasensors work well on salt induced AuNPs aggregation controlled by aptamer adsorption or desorption on AuNPs surface in the presence or absence of TOB. The suggested system was used successfully to detect TOB in chicken eggs and raw milk [49].
6.2.3. Streptomycin (STR)
Streptomycin (Table 1) belongs to the aminoglycoside antibiotic group that is used widely in the veterinary and medical practices to cure infections caused by gram-negative bacteria [100]. Higher accumulation of STR residues results in evolution of pathogenic resistant strains on human tissues after ingestion of infected foodstuffs, which becomes a big risk to livestock and human health [101].
Zhou et al. screened and identified streptomycin aptamer STR1 by affinity magnetic beads-based SELEX which they used to construct an AuNPs-based colorimetric sensor. Competitive binding of STR with its aptamer brings out AuNPs aggregation in salt solution and a visible color change. The sensor showed high affinity and specificity for STR detection in honey samples [70].
Liu et al. isolated a high affinity A15 aptamer to detect STR after 10 SELEX rounds (via affinity chromatograph). Aptamer sequences were used to construct aptasensor based on AuNPs aggregation method. The technique is used to detect antibiotics and other small molecules in food industries [71].
A point of care testing (POCT) STR detection sensor using AuNPs labelled PV (as Nano tracers) and SSB labeled porous silica (as capture probes) and exonuclease-assisted target recycling (for signal amplification) was developed by Luan et al. The system depends on the higher affinity of aptamer towards STR than SSB (single stranded DNA binding protein). When exonuclease I and STR are added, the nano tracer combines with STR to form STR/Apt-Au-PV complex. After this, Exo I keep on digesting the aptamer attached to the complex, and STR is released again to take part in a new cycle. The sensor generates more nano tracers into the solution to boost the sensitivity (Figure 3C) [72].
Zhao et al. reported peroxidase-mimicking activity of AuNPs and their behavior towards the STR-apt complex. AuNPs might oxidize peroxidase substrate in the presence of H2O2. The system is easy to fabricate for large-scale applications. It is rapid, accurate, and can be used to detect a wide range of other small molecules by replacing their particular aptamer sequences [73].
A colorimetric and fluorescence quenching STR detection aptasensor based on double-stranded DNA (dsDNA) and aqueous AuNPs was designed by Emrani et al. In samples lacking STR, aptamer/FAM-labeled CS forms a stable dsDNA, leaving behind salt aggregated AuNPs with a color change in the solution. Contrarily, aptamer binds to STR, while CS stabilizes the AuNP hence no color change. Sensors worked well in serum and milk samples [74].
6.2.4. Neomycin B (NB)
Neomycin (Table 1) is an aminoglycoside, derived from streptomyces fradiae, partially active against Gram-positive bacteria and shows excellent results for Gram-negative bacteria. NB is being widely used in the recent decade to cure gastrointestinal infections of poultry, goats, pigs, sheep, cattle and through intramammary administration to cure mastitis [102]. However, an overdose of neomycin is potentially well-known for its nephrotoxic and ototoxic effects in animals and humans [103]. Thus, it is essential to introduce sufficiently sensitive strategies for neomycin residue detection animal-derived foodstuffs. Unfortunately, only one aptasensor has been reported for neomycin B detection so far.
Khavani, Izadyar et al. theoretically designed high affinity RNA aptamers for NB by employing molecular dynamic (MD) simulations which they used to fabricate AuNP based aptasensor to compare the affinity with a wild type aptamer (AP-W). The resulting LOD of AP-M18 was considerably lower than those of other aptamers suggesting its efficiency as a high affinity aptamer for NB detection [75].
6.3. Anthracyclines and Their Residue Detection
Anthracycline antibiotics could inhibit DNA replication by inhibition and intercalation of topoisomerases. Clinically, they are used in tumor therapy but their long-term use can have severe side effects such as heart failure [19]. The basic structure, common in all anthracyclines, is shown in green (Table 1).
Daunomycin (Table 1) belongs to the anthracycline group of antibiotics are widely used as cancer chemotherapeutic agents. However, DNR is a major carcinogenic, nephrotoxic and mutagenic itself. It could reach drinking water and may contaminate soil if its residue level is not controlled, eliminated or degraded [104].
He et al. constructed the only sensitive method to be used for on-site food safety against DNR by aptamer based AuNPs aggregation. Since no modification of AuNPs or aptamers is required, the method is low cost, precise and has only 5 min reflection time [76].
6.4. Chloramphenicol (CAP) and Their Residue Detection
Chloramphenicol (Table 1) is another broad-spectrum group of antibiotics, [105] widely used in aquaculture and animal husbandry for their efficiency in inhibiting bacterial growth. However, CAP brings severe side effects on the human hematopoietic and digestive system once their residues are ingested through the nutrition [106]. The excessive accumulation of CAP residues in human blood and animal-derived foodstuffs pose adverse effects on humans, such as gray baby syndrome, aplastic anemia, and leukemia [77].
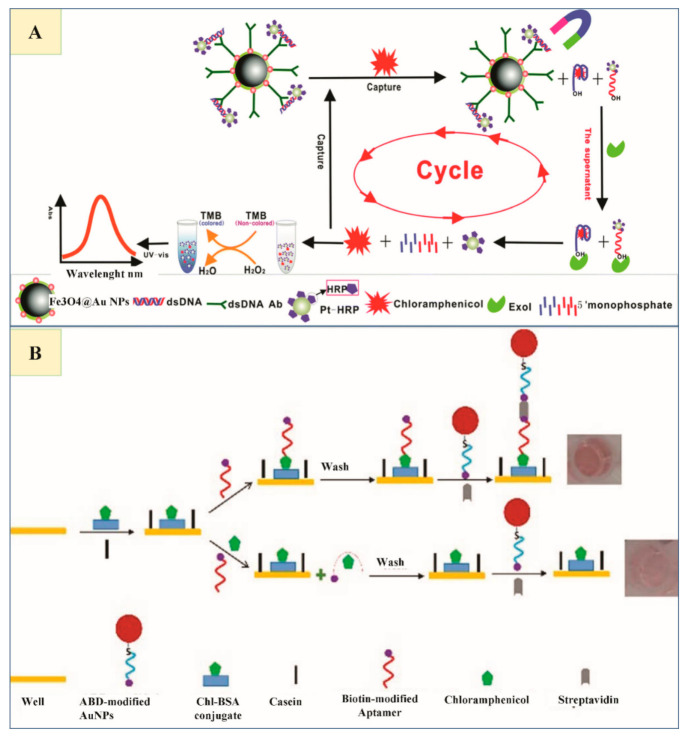
A sensitive, facile and selective method for ultra-trace level residue detection of CAP was devised by Miao et al. which can be efficiently used for the detection of other antibiotics as well by changing the aptamer sequences. Sensor relies on triple signal amplification approach with an Exo I-assisted target recycling and magnetic aptamer-HRP co-immobilized PtNPs nano tracers. This biosensor preparation is complicated (Figure 4A) [78].
Figure 4.
(A) Illustration of the detection processes related to color change based on chloramphenicol (CAP) presence or absence (Reproduced with permission from [78] © 2015 Royal Society of Chemistry). (B) Schematics showing CAP detection based on AuNPs colorimetric method. In the absence of target, the sandwich aptasensor results in bright red color. In the presence of CAP, modified AuNPs cannot bind to the well, with a subsequent pale red color change (Reproduced with permission from [77] © 2016 Elsevier).
Abnous et al. introduced a sandwich aptasensor for CAP detection, based on an indirect competitive enzyme-free setup using biotin, AuNPs and streptavidin. The sandwich structure forms in aptasensor when the sample lacks CAP residues, displaying a sharp red color. On CAP addition, functionalized AuNPs fail to bind 96-well plates, characterized by a faint red color (Figure 4B) [77].
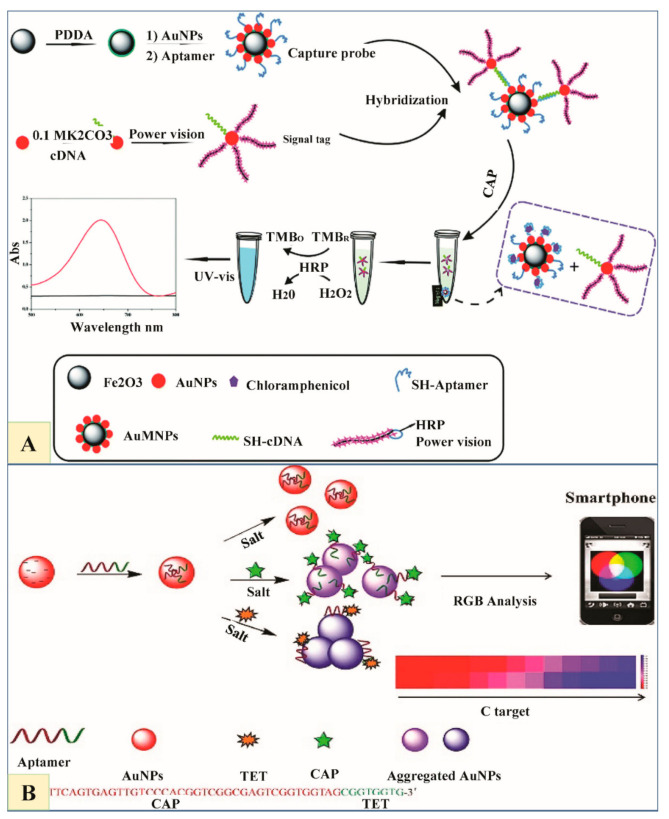
PV labeled AuNPs were modified by conjugation with cDNA (cDNA–AuNPs–PV) as signal amplifiers for CAP detection by Gao et al. They then functionalized a CAP aptamer by immobilizing it on Fe3O4@Au magnetic NPs (AuMNPs–Apt) as capture probe. Special tags might hybridize with cDNA and aptamer to form AuMNP– Apt/cDNA–AuNP–PV conjugates. When CAP is added, it binds to aptamer, dissociating some cDNA–AuNPs–PV on the conjugates with magnetic separation. At this point, PV is catalyzing 3,3’5,5’-tetramethylbenzidine (TMB), which brings out a color change that can be quantified by (UV-vis) spectroscopy (Figure 5A) [79].
Figure 5.
(A) Scheme showing the proposed working principle of CAP detection using cDNA–AuNPs–PV as a signal tag (Reproduced with permission from [79] © 2015 Royal Society of Chemistry). (B) Schematic diagram exhibiting TET/CAP detection in which the multi-Aptamer acts as a molecular switch adjusting the AuNPs aggregation. When a specific target removes the fragment of its particular Apt from the AuNPs surface, unbalanced AuNPs aggregation occurs at different scales under high-salt conditions, causing colloidal color changes, which can be detected by UV-spectrum and Smartphone analysis, respectively (Reproduced with permission from [81] © 2020 Elsevier).
An aptasensor for CAP ultra-trace residue detection based on the use of a new enzyme-polymer nano tracer (as a signal amplifier) was developed by Miao et al. HRP-Au composite was labelled by enzyme-linked polymer (EV) which could efficiently catalyze TMB oxidation via H2O2, causing visible color changes. This simple and sensitive assay find excellent applications in food industries and in situ food safety especially in fish samples [107].
Huang et al. combined aptamer and peroxidase-mimicking DNAzyme-functionalized AuNP nanoprobe for sensitive and rapid CAP detection. They prepared nanoprobe by the sulfhydrylation assembly of cDNA against CAP aptamer and high content hemin/G-quadruplex DNAzyme on the surface of AuNPs. Because of the high efficiency of the nanoprobe signal amplification, the sensitivity of this method rivals several electrochemical biosensors [80].
Javidi et al. developed a CAP detection method in milk using intact long-oligonucleotide aptamers with aptamer terminal locks (ATL). In the ATL approach, long aptamers were used as molecular recognition probes while short-sequences worked as locker probes (LP) which must be complementary to aptamer terminal fragments. The method relies on the interaction of ssDNA (LP) with AuNPs, target-induced release of LP from long aptamer and no interaction of ATL with AuNPs [82].
A biosensor for CAP detection method, in which ssDNA-functionalized AuNPs aggregation was supported by lanthanum (La3+) ions and smartphone imaging was described by Wu et al. However, La3+ as a trigger agent might limit the on-site application because of poor biocompatibility and storage instability. Moreover, the sensitivity and specificity of the analytical performance needs improvement for future studies [51] (Table 3).
Wu, Huang and Wu later devised another protocol for multiplex antibiotics (CAP and TET) detection, based on multifunctional ss-DNA apt coordinately controlling AuNPs aggregation. When one analyte is added into the solution separately, that specific recognized sequence of aptamer binds to it and is dissociated from AuNPs, whereas the non-specific, unrecognized part coordinately controls the salt-induced aggregation of AuNPs [81] (Figure 5B).
6.5. Fluoroquinolones (FQs) and Their Residue Detection
Fluoroquinolones belong to a synthetic group of antibiotics, originated from quinolone nalidixic acid by the addition of a fluorine atom at carbon 6 and piperazine at carbon 7 position [108]. FQs kill bacteria or inhibit their growth by inhibiting their DNA replication favored by their chemical structure [109] (Table 1). Since the invention of FQs in the 1970s, and their use in livestock and human therapeutics in the 1990s, they became commonly-prescribed antibiotics for their broad-spectrum mode of action towards Gram-negative, Gram-positive bacteria and mycoplasma [110]. However, due to their widespread use, they can easily enter the environmental water table by human defecation into the sewage system or spreading of compost onto the agricultural lands, which may cause food-borne bacterial emergence in humans or severe allergic reactions [111].
Ciprofloxacin belongs to FQs, used to cure various bacterial infections such as respiratory infections, gastrointestinal diseases and urinary tract infections. Being a broad-spectrum antibiotic, CIP is highly prescribed to be used for livestock and humans [112]. It can enter the drinking water and environment in a number of ways, including pharmaceutical production emissions, domestic sewage, solid waste disposal, landfill dumping, and livestock manure, etc. [113]. CIP has been detected at a considerably higher concentration in river waters [114].
Lavaee et al. constructed the only CIP detection assay based on modified AuNPs with cDNA and CIP aptamer. Flower-shaped modified AuNPs surface show enzyme-like activity which catalyzes nitrophenol reduction by NaBH4. The method completes in one hour and is used broadly to detect CIP in real samples such as milk, water, and serum [83].
6.6. Tetracyclines and Their Residue Detection
Tetracyclines are a broad-spectrum group of compounds employed commonly because of their low cost in comparison to other antibiotics. At present, more than 20 different tetracyclines are available on the market; however, oxytetracycline, doxycycline, tetracycline, and chlortetracycline are among broadly used in veterinary medicine [115]. Their name is derived from their basic molecular structure (Table 1) comprised of six linearly arranged rings [19]. Excessive use of TET as antibiotics and a growth promoter in veterinary medicine is causing its residue accumulation in foodstuffs, which is a grave risk for human health and the environment [116].
6.6.1. Oxytetracyclines (OTC).
OTCs are the major antibacterial agents, inhibiting the bacterial protein synthesis by binding to 30 S ribosomal subunit reversibly. However, the overuse of these drugs is causing serious problems because of their residue accumulation in animal derived foods, especially milk, which is directly toxic or can cause severe allergic reactions to many hypersensitive individuals [117].
Kim et al. employed a high specificity OTC binding ssDNA aptamer that can induce unmodified AuNPs aggregation by desorption from their surface resulting from the target-aptamer interactions. The protocol can be optimized for other unmodified AuNPs-based aptasensors [84].
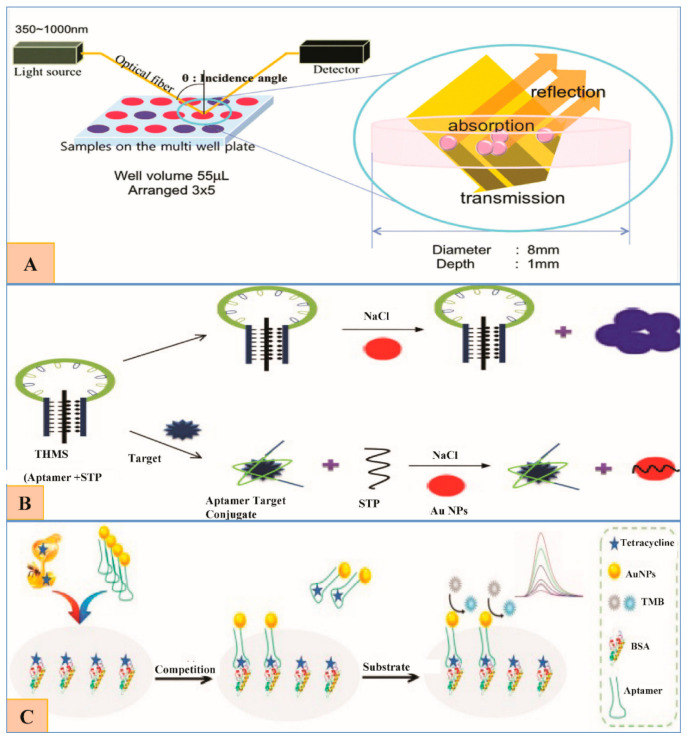
A reflectance-based aptesensor that operates at two wavelengths (520 and 650 nm) for OTC detection was devised by Seo et al. They found that their sensor generates more sensitive and stable signals as compared to the absorbance-based assays based on AuNPs aggregation even at higher concentrations (Figure 6A). They considered it a better approach for the portable detection of such small molecules by using aptamers [50].
Figure 6.
(A) The schematics showing the reflectance-based multi-well plate colorimetric aptasensor using AuNPs (Reproduced with permission from [50] © 2015 Royal Society of Chemistry). (B) Schematic explanation of TET detection based on colorimetric triple-helix molecular switch (THMS). In the absence of Tetracycline (TET), THMS (Aptamer + STP) remains stable, resulting in AuNPs aggregation by salt, color changes from red to blue. In the presence of TET, aptamer binds to its target, the signal transduction probe (STP) leaves the THMS and adsorbs on the surface of AuNPs thus stabilizing them, so no color change (Reproduced with permission from [87] © 2015 Elsevier). (C). Illustration of the proposed gold nanoparticle-linked competition-based aptamer assay (Reproduced with permission from [89] © 2020 MDPI).
A low cost, rapid, high selectivity and convenient DNA aptasensor for KAN and OTC was constructed by Xu et al. After the occurrence of recognition events, DNA sequences released from MB surface and DNA probes captured HRP modified AuNPs. Consequently, o-phenylenediamine and 3,3′,5,5′-tetramethylbenzidine were oxidized by horseradish peroxidase displayed color changes [85].
Kazerooni et al. designed an OTC aptasensor based on salt-induced AuNPs aggregation by OTC aptamer interaction in the presence or absence of target. The sensor is used in the field of food safety and biomedicine as a promising tool and can be extended for other antibiotics by replacing aptamer sequences [5].
6.6.2. Tetracycline (TET)
The TET misuse in aquaculture and animal husbandry results in the accumulation of its residues at higher levels in environment, such as water and soil [118]. Owing to its life-threatening effects, it is very important to detect the trace levels in an effective and efficient manner (Table 1 for TET structure).
He et al. proposed a TET detection strategy based on the aggregation of AuNPs, controlled by TET aptamer interaction with TET and hexadecyltrimethylammonium bromide (CTAB). CTAB is a surfactant to control the shape and size of AuNPs and form a complex with aptamer in the absence of target, thus aggregating AuNPs and bringing out a significant color change [86].
He et al. introduced a simple TET detection method based on the AuNPs aggregation controlled by TET, aptamer and poly diallyldimethylammonium (PDDA). Cationic polymer PDDA controls AuNPs aggregation and aptamer hybridization. TET addition reduces aptamers to form an aptamer-TET complex; thus, cationic polymers aggregate AuNPs and bring out a significant color change [119].
A fast, selective and sensitive TET detection (in blood serum and milk) aptasensor was designed by Ramezani et al. based on AuNPs and triple-helix molecular switch (THMS). THMS exhibits numerous advantages including preserving high selectivity, original aptamer affinity, stability, and sensitivity. THMS system commonly comprises a label-free target-specific aptamer, a dual-labeled oligonucleotide as a signal transduction probe (STP) and two arm segments (Figure 6B) [87].
A specific and sensitive TET detection system was documented by Luo et al. using negatively-charged TET aptamer and cysteamine-stabilized AuNPs (CS-AuNPs) probe which is positively-charged. The method is particularly useful for real-time and on-site TET detection in milk [88].
In a recent study by Sheng, Liang et al., TET-BSA were coupled by glutaraldehyde crosslinking assay and applied to the microplate for competitive binding (of free TET in sample and fixed TET in microplate) with aptamer. Concurrently, TET aptamer-labeled AuNPs (TET apt-AuNPs) were prepared. In this way, a novel competitive TET detection sensor for honey was developed based on greater catalysis ability of AuNPs and higher aptamer selectivity (Figure 6C) [89].
6.7. Sulfonamides (SAs) and Their Residue Detection
SAs (Table 1) are one of the main antibiotic groups that are extensively used in human medicine, aquaculture, and livestock husbandry. SAs are recently detected far and wide in the aquatic ecosystem, posing health risks to living organisms [120]. SA residues in foodstuffs envisioned for human ingestion is a world-wide apprehension since they are evolving antibiotic resistance, allergic for humans and carcinogenic [121]. The hydrogen atom on sulfamine is usually substituted by a variety of heterocyclic rings to make different kinds of sulfonamides [122].
Sulfadimethoxine (SDM)
SDM (Table 1), is a major class of livestock and veterinary importance antibiotics, used widely for the management of coccidiosis [123]. It is used as feed additive for the prevention and treatment of various animal diseases because of its broad-spectrum antibacterial activity. However, the misuse of SDM is associated to severe health hazards; for example, acute haemolytic anemia [124]. Consequently, concerns for SDM residue detection in animal-derived food, especially milk, honey, eggs, meat and other foodstuffs, have been raised.
A fast, label-free and simple SDM detection sensor was optimized by Chen et al. based on controlling the aptamers to AuNPs relative amount, ionic strength by salt addition and the pH value of AuNPs solution [90].
Yan et al. reported a reliable, cost-effective and sensitive colorimetric sensor for SDM detection relies on peroxidase-like catalytic activity of AuNPs. SDM-apt undergoes desorption from AuNPs surface in the presence of SDM which reactivates the catalytic activity of AuNPs. Color change is dependent on SDM concentration and can be seen by UV-visible absorptiometry at 650 nm [91].
Chen at al. recently devised a label-free, dichromatic sensor for SDM detection. Dichromatic mode was achieved by AuNPs color changing and fluorescence at 530 nm. SDM-apt dissociate from the AuNP surface in the presence of SDM to make an aptamer target complex which shows color changes from in the solution and fluorescence by adding syber green I and cDNA [92].
7. A Generalized Overview, Future Perspective, Challenges and Conclusions
Because the development of innovative or emerging methods for accurate antibiotic detection is an environmental and clinical need, we gave a comprehensive overview of the reported aptasensor advances over the past decade. Some of the aforementioned aptasensors are already being used at commercial level by food industries to detect antibiotic residues for food safety. Some new methods are still in theoretical and early testing phase with a high potential for marketing in the future. Colorimetric aptasensors are highly advantageous as the experimental results can be directly observed/quantified by analyzing visual color changes via naked eye, spectrophotometer or mobile phone chromatism without any kind of complex equipment/instrumentation. Mostly, 5’-end of the different aptamer sequences were used, preferably for immobilization purposes. The specificity of the used aptamer sequences towards their analytes (in many protocols) was verified by testing their sensitivity against possible interfering or structurally similar substances. Nanotechnology made a significant contribution in the field of biosensing; e.g., combining particular aptamer sequences with different nanomaterials led to the development of highly selective and sensitive aptasensors. There are numerous similarities and differences between various sensing protocols as described previously in this review such as, all colorimetric aptasensors discussed here, involves the use of a sensing or recognition material (aptamer) and a colorimetric agent (AuNPs) for signal transduction. From the sensing viewpoint, the inherent properties of AuNPs also offer numerous benefits for the construction of smart sensors, e.g., surface adsorption, dynamic light scattering with the change in their particle size, and the salt-induced aggregation of the AuNPs. However, not all of these aptasensors have the same recognition systems or obey the same working principle (already described for several reports). Subsequently, these kinds of portable devices are simple to operate, rapid, inexpensive, and suitable for on-site detection applications with high sensitivity, selectivity and flexibility. However, some limitations and challenges still need to be addressed here.
All of the protocols used DNA aptamer sequences. RNA sequences should also be characterized for their use in this kind of detection methods. Most of the reported aptasensors are intended to be extended for the detection of a number of different low molecular weight analytes (other antibiotics) by changing the particular aptamer sequence with the most suitable one against the other intended targets to be detected. Unfortunately, not enough high-affinity aptamer sequences have been developed to be replaced for an already existing protocol. We have cited 40 different protocols reported over the decade so far, with most of the studies using the same once-developed aptamer sequence for a specific analyte again and again in different protocols. Despite numerous advances currently made in the field of aptamer based biosensing platform, they are still considered immature if we compare them with immunosensors. This may be attributed to a limited high efficiency aptamer variety available at present. Screening of class specific aptamers to detect common group members can also be helpful to recognize more antibiotic types. Thus, there is an urgent need for more high affinity aptamer sequences to be screened for a wide range of antibiotics (small molecular targets) in the future. Aptamer immobilization approaches, especially their bioconjugation with AuNPs or other nanomaterials, may sometimes confine the aptamer’s target recognition capacity as there is relatively less knowledge regarding the surface-immobilization techniques for aptamers. There is an increasing demand to develop some novel modification and immobilization strategies concerning greater nanomaterials biocompatibility so that the aptamers may attach easily to their functional signal reporters. Because the on-site detection of the antibiotic targets is challenging, it needs to be given more attention. Additionally, environmental or food sample (to be analyzed) conditions, e.g., viscosity, ionic strength, pH, as well as non-specific interactions of the sample matrix with aptamer, must be considered.
Further studies are required to investigate the competing ligand interference, in other words to address the false-negative/ false-positive results or to confirm the selectivity of the aptasensor against their specific target antibiotic. Most importantly, attention is needed to fill the gap of certain parameters (such as pH, temperature, ionic strength, etc.) for the compatibility between aptasensor design and aptamer selection (selection conditions should be same) so as to improve the identification capacity of the aptamer which may indirectly enhance the performance of the sensor in terms of stability, specificity, affinity and sensitivity. Besides this, some external factors may also easily influence the colorimetric assays such as the color of the sample and background fluorescence. Some other challenges are associated with longer fabrication time duration and poor stability or reuse.
By considering the significant advances in the above-mentioned directions, we aim to inspire more efforts for the development of AuNPs based colorimetric aptasensors for on-site detection of antibiotics and other small molecule pollutants. In the end, we would like to recommend improvements by combining various highly efficient AuNPs-based colorimetric aptasensor strategies on one platform to allow multiple residue detections from the only sample as a source. Overall, although there is still a long way to go, we are hopeful that aptamer-based biosensing methods, especially AuNPs calorimetry, will ultimately be proved to be a real-world tool, meeting the present and future challenges which might be impossible otherwise, with the latest available methods. Thus, more efforts are required to manufacture more commercially available aptasensors with high efficiency, easy operation, and low-cost.
Fast and precise assays for the detection of antibiotics still face certain limitations such as sensitivity, specificity, linearity, turnaround time, cost effectiveness, health risks, etc. Nanotechnology is believed to be key to answering these questions while minimizing systemic toxicity. Aptamers offer several advantages over other materials, such as high binding specificity and physicochemical stability, simple modification, long shelf life, minor variation, slight denaturation susceptibility, and cost effectiveness. They interact with a variety of molecules including antibiotics. The enzyme-like activities of gold nanoparticles potentiate their role in biosensing systems and opens the road for their application in various fields of scientific research, diagnostics, and therapy. These superiorities motivate the development of aptasensors for wider applications.
Abbreviations
| AuNPs | gold nanoparticles |
| BSA | bovine serum albumin |
| BRE | bio-recognition element |
| cDNA | complementary DNA |
| CS | complementary strand |
| dsDNA | double stranded DNA |
| KD | dissociation constant |
| LOD | limit of detection |
| SELEX | systematic evolution of ligands by exponential enrichment |
| SPR | surface plasmon resonance spectroscopy |
| STE | Signal transduction element |
| SSB | ssDNA binding protein |
| ssDNA | single stranded DNA |
| THMS | triple-helix molecular switch |
Funding
This research was funded by the Fundamental Research Funds for the Central Universities of China, grant number (YD2070002013).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abedalwafa M.A., Li Y., Ni C., Wang L. Colorimetric sensor arrays for the detection and identification of antibiotics. Anal. Methods. 2019;11:2836–2854. doi: 10.1039/C9AY00371A. [DOI] [Google Scholar]
- 2.Gould I.M., Bal A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence. 2013;4:185–191. doi: 10.4161/viru.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventola C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015;40:277. [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention, Office of Infectious Disease . Antibiotic Resistance Threats in the United States, 2013. Center for Disease Control and Prevention; Atlanta, GA, USA: 2013. [Google Scholar]
- 5.Kazerooni H., Bahreyni A., Ramezani M., Abnous K., Taghdisi S.M. A colorimetric aptasensor for selective detection of oxytetracycline in milk, using gold nanoparticles and oxytetracline-short aptamer. Nanomed. J. 2019;6:105–111. [Google Scholar]
- 6.Song Y., Duan F., Zhang S., Tian J.-Y., Zhang Z., Wang Z.-W., Liu C.-S., Xu W.-M., Du M. Iron oxide @ mesoporous carbon architectures derived from an Fe(II)-based metal organic framework for highly sensitive oxytetracycline determination. J. Mater. Chem. A. 2017;5:19378–19389. doi: 10.1039/C7TA03959J. [DOI] [Google Scholar]
- 7.Briscoe S.E., McWhinney B.C., Lipman J., Roberts J.A., Ungerer J.P. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J. Chromatogr. B. 2012;907:178–184. doi: 10.1016/j.jchromb.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Dai X.-H., Xue Y.-G., Liu H.-J., Dai L.-L., Yan H., Li N. Development of determination method of fluoroquinolone antibiotics in sludge based on solid phase extraction and HPLC-fluorescence detection analysis. Huan Jing Ke Xue. 2016;37:1553–1561. [PubMed] [Google Scholar]
- 9.Meersche T.V.D., Van Pamel E., Van Poucke C., Herman L., Heyndrickx M., Rasschaert G., Daeseleire E. Development, validation and application of an ultra high performance liquid chromatographic-tandem mass spectrometric method for the simultaneous detection and quantification of five different classes of veterinary antibiotics in swine manure. J. Chromatogr. A. 2016;1429:248–257. doi: 10.1016/j.chroma.2015.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y.-J., Wu H.-L., Fu H.-Y., Zhao J., Li Y.-N., Li S.-F., Kang C., Yu R.-Q. Chromatographic background drift correction coupled with parallel factor analysis to resolve coelution problems in three-dimensional chromatographic data: Quantification of eleven antibiotics in tap water samples by high-performance liquid chromatography coupled with a diode array detector. J. Chromatogr. A. 2013;1302:72–80. doi: 10.1016/j.chroma.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Berendsen B.J., Wegh R.S., Memelink J., Zuidema T., Stolker L.A. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta. 2015;132:258–268. doi: 10.1016/j.talanta.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Carballo E., González-Barreiro C., Scharf S., Gans O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007;148:570–579. doi: 10.1016/j.envpol.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Haller M.Y., Müller S.R., McArdell C.S., Alder A.C., Suter M.J.-F. Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography–mass spectrometry. J. Chromatogr. A. 2002;952:111–120. doi: 10.1016/S0021-9673(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 14.Gbylik-Sikorska M., Posyniak A., Sniegocki T., Zmudzki J. Liquid chromatography–tandem mass spectrometry multiclass method for the determination of antibiotics residues in water samples from water supply systems in food-producing animal farms. Chemosphere. 2015;119:8–15. doi: 10.1016/j.chemosphere.2014.04.105. [DOI] [PubMed] [Google Scholar]
- 15.Han Q., Wang R., Xing B., Chi H., Wu D., Wei Q. Label-free photoelectrochemical aptasensor for tetracycline detection based on cerium doped CdS sensitized BiYWO6. Biosens. Bioelectron. 2018;106:7–13. doi: 10.1016/j.bios.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 16.Yang S., Zhu X., Wang J., Jin X., Liu Y., Qian F., Zhang S., Chen J. Combustion of hazardous biological waste derived from the fermentation of antibiotics using TG–FTIR and Py–GC/MS techniques. Bioresour. Technol. 2015;193:156–163. doi: 10.1016/j.biortech.2015.06.083. [DOI] [PubMed] [Google Scholar]
- 17.Pauter K., Szultka-Młyńska M., Buszewski B. Determination and identification of antibiotic drugs and bacterial strains in biological samples. Molecules. 2020;25:2556. doi: 10.3390/molecules25112556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mungroo N.A., Neethirajan S. Biosensors for the detection of antibiotics in poultry industry—A Review. Biosensors. 2014;4:472–493. doi: 10.3390/bios4040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehlhorn A., Rahimi P., Joseph Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors. 2018;8:54. doi: 10.3390/bios8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q., Yang Q., Wu W. Progress on Structured Biosensors for Monitoring Aflatoxin B1 from Biofilms: A Review. Front. Microbiol. 2020;11:408. doi: 10.3389/fmicb.2020.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R.E., Zhang Y., Cai J., Cai W., Gao T. Aptamer-based fluorescent biosensors. Curr. Med. Chem. 2011;18:4175–4184. doi: 10.2174/092986711797189637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Z., Zhou H., Jiang H., Ou H., Li X., Zhang L. Development of a fraction collection approach in capillary electrophoresis SELEX for aptamer selection. Analyst. 2015;140:2664–2670. doi: 10.1039/C5AN00183H. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer F., Tolle F., Rosenthal M., Brändle G.M., Ewers J., Mayer G. Identification and characterization of nucleobase-modified aptamers by click-SELEX. Nat. Protoc. 2018;13:1153–1180. doi: 10.1038/nprot.2018.023. [DOI] [PubMed] [Google Scholar]
- 24.Liu D., Zhang Z., Yin Y., Jia F., Wu Q., Tian P., Wang D. Development and evaluation of a novel in situ target-capture approach for aptamer selection of human noroviruses. Talanta. 2019;193:199–205. doi: 10.1016/j.talanta.2018.09.084. [DOI] [PubMed] [Google Scholar]
- 25.Sabri M.Z., Hamid A.A.A., Hitam S.M.S., Rahim M.Z.A. In-silico selection of aptamer: A review on the revolutionary approach to understand the aptamer design and interaction through computational chemistry. Mater. Today Proc. 2019;19:1572–1581. doi: 10.1016/j.matpr.2019.11.185. [DOI] [Google Scholar]
- 26.Ahirwar R., Nahar S., Aggarwal S., Ramachandran S., Maiti S., Nahar P. In silico selection of an aptamer to estrogen receptor alpha using computational docking employing estrogen response elements as aptamer-alike molecules. Sci. Rep. 2016;6:21285. doi: 10.1038/srep21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y., Zhu Z., An Y., Zhang W., Zhang H., Liu D., Yu C., Duan W., Yang C.J. Selection of DNA Aptamers against Epithelial Cell Adhesion Molecule for Cancer Cell Imaging and Circulating Tumor Cell Capture. Anal. Chem. 2013;85:4141–4149. doi: 10.1021/ac400366b. [DOI] [PubMed] [Google Scholar]
- 28.Wolter O., Mayer G. Aptamers as valuable molecular tools in neurosciences. J. Neurosci. 2017;37:2517–2523. doi: 10.1523/JNEUROSCI.1969-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomilin F.N., Moryachkov R., Shchugoreva I., Zabluda V.N., Peters G., Platunov M., Spiridonova V., Melnichuk A., Atrokhova A., Zamay S.S., et al. Four steps for revealing and adjusting the 3D structure of aptamers in solution by small-angle X-ray scattering and computer simulation. Anal. Bioanal. Chem. 2019;411:6723–6732. doi: 10.1007/s00216-019-02045-0. [DOI] [PubMed] [Google Scholar]
- 30.Sun H., Zu Y. A highlight of recent advances in aptamer technology and its application. Molecules. 2015;20:11959–11980. doi: 10.3390/molecules200711959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao H., Tian Y., Zhang M., Liu J., Yuan Y., Tan J., Ma A. Selection, identification, and application of aptamers against Agaricus bisporus lectin to establish an aptamer-AuNPs colorimetric method for detection of ABL. J. Food Qual. 2020;2020 doi: 10.1155/2020/8821295. [DOI] [Google Scholar]
- 32.Li L., Xu S., Yan H., Li X., Yazd H.S., Li X., Huang T., Cui C., Jiang J., Tan W. Nucleic acid aptamers for molecular diagnostics and therapeutics: Advances and perspectives. Angew. Chem. Int. Ed. 2020;60:2221–2231. doi: 10.1002/anie.202003563. [DOI] [PubMed] [Google Scholar]
- 33.Kalra P., Dhiman A., Cho W.C., Bruno J.G., Sharma T.K. Simple methods and rational design for enhancing aptamer sensitivity and specificity. Front. Mol. Biosci. 2018;5:41. doi: 10.3389/fmolb.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez F.J., Ozalp V.C. Graphene and other nanomaterial-based electrochemical aptasensors. Biosensors. 2012;2:1–14. doi: 10.3390/bios2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armbruster D., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008;29:S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 36.Eggins B.R. Chemical Sensors and Biosensors. Volume 28 John Wiley & Sons; Hoboken, NJ, USA: 2008. [Google Scholar]
- 37.Song S., Wang L., Li J., Fan C., Zhao J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008;27:108–117. doi: 10.1016/j.trac.2007.12.004. [DOI] [Google Scholar]
- 38.Lim Y.C., Kouzani A.Z., Duan W. Aptasensors: A Review. J. Biomed. Nanotechnol. 2010;6:93–105. doi: 10.1166/jbn.2010.1103. [DOI] [PubMed] [Google Scholar]
- 39.Feng C., Dai S., Wang L. Optical aptasensors for quantitative detection of small biomolecules: A review. Biosens. Bioelectron. 2014;59:64–74. doi: 10.1016/j.bios.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Robati R.Y., Arab A., Ramezani M., Langroodi F.A., Abnous K., Taghdisi S.M. Aptasensors for quantitative detection of kanamycin. Biosens. Bioelectron. 2016;82:162–172. doi: 10.1016/j.bios.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Zahra Q.U.A., Khan Q.A., Luo Z. Advances in optical aptasensors for early detection and diagnosis of various cancer types. Front. Oncol. 2021;11:19. doi: 10.3389/fonc.2021.632165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holzinger M., Le Goff A., Cosnier S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014;2:63. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma R., Ragavan K., Thakur M., Raghavarao K. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 2015;74:612–627. doi: 10.1016/j.bios.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Mohammadpour A.H., Tavassoli A., Khakzad M.R., Zibaee E., Afshar M., Hashemzaei M., Karimi G. Effect of gold nanoparticles on postoperative peritoneal adhesions in rats. Nanomed. J. 2015;2:211–216. [Google Scholar]
- 45.Babaei M., Ganjalikhani M. A systematic review of gold nanoparticles as novel cancer therapeutics. Nanomed. J. 2014;1:211–219. [Google Scholar]
- 46.Chen H., Zhou K., Zhao G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018;78:83–94. doi: 10.1016/j.tifs.2018.05.027. [DOI] [Google Scholar]
- 47.Kim C.-H., Lee L.-P., Min J.-R., Lim M.-W., Jeong S.-H. An indirect competitive assay-based aptasensor for detection of oxytetracycline in milk. Biosens. Bioelectron. 2014;51:426–430. doi: 10.1016/j.bios.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Xu C., Ying Y., Ping J. Colorimetric aggregation assay for kanamycin using gold nanoparticles modified with hairpin DNA probes and hybridization chain reaction-assisted amplification. Microchim. Acta. 2019;186:448. doi: 10.1007/s00604-019-3574-7. [DOI] [PubMed] [Google Scholar]
- 49.Ma Q., Wang Y., Jia J., Xiang Y. Colorimetric aptasensors for determination of tobramycin in milk and chicken eggs based on DNA and gold nanoparticles. Food Chem. 2018;249:98–103. doi: 10.1016/j.foodchem.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 50.Seo H.B., Kwon Y.S., Lee J.E., Cullen D., Noh H.M., Gu M.B. A novel reflectance-based aptasensor using gold nanoparticles for the detection of oxytetracycline. Analyst. 2015;140:6671–6675. doi: 10.1039/C5AN00726G. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y.-Y., Liu B.-W., Huang P., Wu F.-Y. A novel colorimetric aptasensor for detection of chloramphenicol based on lanthanum ion–assisted gold nanoparticle aggregation and smartphone imaging. Anal. Bioanal. Chem. 2019;411:7511–7518. doi: 10.1007/s00216-019-02149-7. [DOI] [PubMed] [Google Scholar]
- 52.Huang P.-C., Gao N., Li J.-F., Wu F.-Y. Colorimetric detection of methionine based on anti-aggregation of gold nanoparticles in the presence of melamine. Sens. Actuators B Chem. 2018;255:2779–2784. doi: 10.1016/j.snb.2017.09.092. [DOI] [Google Scholar]
- 53.Abdullah S.M., Rachid S. On column binding a real-time biosensor for β-lactam antibiotics quantification. Molecules. 2020;25:1248. doi: 10.3390/molecules25051248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng K., Zhang J., Wang Y., Wang Z.H., Zhang S.X., Wu C.M., Shen J.Z. Development of a rapid multi-residue assay for detecting β-lactams using penicillin binding protein 2x. Biomed. Environ. Sci. 2013;26:100–109. doi: 10.3967/0895-3988.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Lamar J., Petz M. Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices. Anal. Chim. Acta. 2007;586:296–303. doi: 10.1016/j.aca.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 56.Gustavsson E., Degelaen J., Bjurling P., Sternesjo A. Determination of β-lactams in milk using a surface plasmon resonance-based biosensor. J. Agric. Food Chem. 2004;52:2791–2796. doi: 10.1021/jf0344284. [DOI] [PubMed] [Google Scholar]
- 57.Chan P.-H., Liu H.-B., Chen Y.W., Chan K.-C., Tsang C.-W., Leung Y.-C., Wong K.-Y. Rational design of a novel fluorescent biosensor for β-lactam antibiotics from a class a β-lactamase. J. Am. Chem. Soc. 2004;126:4074–4075. doi: 10.1021/ja038409m. [DOI] [PubMed] [Google Scholar]
- 58.Merola G., Martini E., Tomassetti M., Campanella L. New immunosensor for β-lactam antibiotics determination in river waste waters. Sens. Actuators B Chem. 2014;199:301–313. doi: 10.1016/j.snb.2014.03.083. [DOI] [Google Scholar]
- 59.Song K.-M., Jeong E., Jeon W., Cho M., Ban C. Aptasensor for ampicillin using gold nanoparticle based dual fluorescence–colorimetric methods. Anal. Bioanal. Chem. 2012;402:2153–2161. doi: 10.1007/s00216-011-5662-3. [DOI] [PubMed] [Google Scholar]
- 60.Shayesteh O.H., Ghavami R. Two colorimetric ampicillin sensing schemes based on the interaction of aptamers with gold nanoparticles. Microchim. Acta. 2019;186:485. doi: 10.1007/s00604-019-3524-4. [DOI] [PubMed] [Google Scholar]
- 61.Sharma T.K., Ramanathan R., Weerathunge P., Mohammadtaheri M., Daima H.K., Shukla R., Bansal V. Aptamer-mediated ‘turn-off/turn-on’ nanozyme activity of gold nanoparticles for kanamycin detection. Chem. Commun. 2014;50:15856–15859. doi: 10.1039/C4CC07275H. [DOI] [PubMed] [Google Scholar]
- 62.Song K.-M., Cho M., Jo H., Min K., Jeon S.H., Kim T., Han M.S., Ku J.K., Ban C. Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal. Biochem. 2011;415:175–181. doi: 10.1016/j.ab.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Niu S., Lv Z., Liu J., Bai W., Yang S., Chen A. Colorimetric aptasensor using unmodified gold nanoparticles for homogeneous multiplex detection. PLoS ONE. 2014;9:e109263. doi: 10.1371/journal.pone.0109263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ha N.-R., Jung I.-P., Kim S.-H., Kim A.-R., Yoon M.-Y. Paper chip-based colorimetric sensing assay for ultra-sensitive detection of residual kanamycin. Process. Biochem. 2017;62:161–168. doi: 10.1016/j.procbio.2017.07.008. [DOI] [Google Scholar]
- 65.Liu J., Zeng J., Tian Y., Zhou N. An aptamer and functionalized nanoparticle-based strip biosensor for on-site detection of kanamycin in food samples. Analyst. 2017;143:182–189. doi: 10.1039/C7AN01476G. [DOI] [PubMed] [Google Scholar]
- 66.Zhou N., Zhang J., Tian Y. Aptamer-based spectrophotometric detection of kanamycin in milk. Anal. Methods. 2014;6:1569. doi: 10.1039/c3ay41816b. [DOI] [Google Scholar]
- 67.Abedalwafa M.A., Tang Z., Qiao Y., Mei Q., Yang G., Li Y., Wang L. An aptasensor strip-based colorimetric determination method for kanamycin using cellulose acetate nanofibers decorated DNA–gold nanoparticle bioconjugates. Microchim. Acta. 2020;187:1–9. doi: 10.1007/s00604-020-04348-x. [DOI] [PubMed] [Google Scholar]
- 68.Shayesteh O.H., Khosroshahi A.G. A polyA aptamer-based label-free colorimetric biosensor for the detection of kanamycin in human serum. Anal. Methods. 2020;12:1858–1867. doi: 10.1039/D0AY00326C. [DOI] [Google Scholar]
- 69.Han X., Zhang Y., Nie J., Zhao S., Tian Y., Zhou N. Gold nanoparticle based photometric determination of tobramycin by using new specific DNA aptamers. Microchim. Acta. 2018;185:4. doi: 10.1007/s00604-017-2568-6. [DOI] [PubMed] [Google Scholar]
- 70.Zhou N., Wang J., Zhang J., Li C., Tian Y., Wang J. Selection and identification of streptomycin-specific single-stranded DNA aptamers and the application in the detection of streptomycin in honey. Talanta. 2013;108:109–116. doi: 10.1016/j.talanta.2013.01.064. [DOI] [PubMed] [Google Scholar]
- 71.Liu Z., Zhang Y., Xie Y., Sun Y., Bi K., Cui Z., Zhao L., Fan W. An aptamer-based colorimetric sensor for streptomycin and its application in food inspection. Chem. Res. Chin. Univ. 2017;33:714–720. doi: 10.1007/s40242-017-7029-6. [DOI] [Google Scholar]
- 72.Luan Q., Miao Y., Gan N., Cao Y., Li T., Chen Y. A POCT colorimetric aptasensor for streptomycin detection using porous silica beads- enzyme linked polymer aptamer probes and exonuclease-assisted target recycling for signal amplification. Sensors Actuators B Chem. 2017;251:349–358. doi: 10.1016/j.snb.2017.04.149. [DOI] [Google Scholar]
- 73.Zhao J., Wu Y., Tao H., Chen H., Yang W., Qiu S. Colorimetric detection of streptomycin in milk based on peroxidase-mimicking catalytic activity of gold nanoparticles. RSC Adv. 2017;7:38471–38478. doi: 10.1039/C7RA06434A. [DOI] [Google Scholar]
- 74.Emrani A.S., Danesh N.M., Lavaee P., Ramezani M., Abnous K., Taghdisi S.M. Colorimetric and fluorescence quenching aptasensors for detection of streptomycin in blood serum and milk based on double-stranded DNA and gold nanoparticles. Food Chem. 2016;190:115–121. doi: 10.1016/j.foodchem.2015.05.079. [DOI] [PubMed] [Google Scholar]
- 75.Khavani M., Izadyar M., Housaindokht M.R. Theoretical design and experimental study on the gold nanoparticles based colorimetric aptasensors for detection of neomycin B. Sens. Actuators B Chem. 2019;300:126947. doi: 10.1016/j.snb.2019.126947. [DOI] [Google Scholar]
- 76.He L., Zhi W., Wu Y., Zhan S., Wang F., Xing H., Zhou P. A highly sensitive resonance scattering based sensor using unmodified gold nanoparticles for daunomycin detection in aqueous solution. Anal. Methods. 2012;4:2266–2271. doi: 10.1039/c2ay25596k. [DOI] [Google Scholar]
- 77.Abnous K., Danesh N.M., Ramezani M., Emrani A.S., Taghdisi S.M. A novel colorimetric sandwich aptasensor based on an indirect competitive enzyme-free method for ultrasensitive detection of chloramphenicol. Biosens. Bioelectron. 2016;78:80–86. doi: 10.1016/j.bios.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 78.Miao Y., Gan N., Ren H.-X., Li T., Cao Y., Hu F., Yan Z., Chen Y. A triple-amplification colorimetric assay for antibiotics based on magnetic aptamer–enzyme co-immobilized platinum nanoprobes and exonuclease-assisted target recycling. Analyst. 2015;140:7663–7671. doi: 10.1039/C5AN01142F. [DOI] [PubMed] [Google Scholar]
- 79.Gao H., Gan N., Pan D., Chen Y., Li T., Cao Y., Fu T. A sensitive colorimetric aptasensor for chloramphenicol detection in fish and pork based on the amplification of a nano-peroxidase-polymer. Anal. Methods. 2015;7:6528–6536. doi: 10.1039/C5AY01379H. [DOI] [Google Scholar]
- 80.Huang W., Zhang H., Lai G., Liu S., Li B., Yu A. Sensitive and rapid aptasensing of chloramphenicol by colorimetric signal transduction with a DNAzyme-functionalized gold nanoprobe. Food Chem. 2019;270:287–292. doi: 10.1016/j.foodchem.2018.07.127. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y.-Y., Huang P., Wu F.-Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2020;304:125377. doi: 10.1016/j.foodchem.2019.125377. [DOI] [PubMed] [Google Scholar]
- 82.Javidi M., Housaindokht M.R., Verdian A., Razavizadeh B.M. Detection of chloramphenicol using a novel apta-sensing platform based on aptamer terminal-lock in milk samples. Anal. Chim. Acta. 2018;1039:116–123. doi: 10.1016/j.aca.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 83.Lavaee P., Danesh N.M., Ramezani M., Abnous K., Taghdisi S.M. Colorimetric aptamer based assay for the determination of fluoroquinolones by triggering the reduction-catalyzing activity of gold nanoparticles. Microchim. Acta. 2017;184:2039–2045. doi: 10.1007/s00604-017-2213-4. [DOI] [Google Scholar]
- 84.Kim Y.S., Kim J.H., Kim I.A., Lee S.J., Jurng J., Gu M.B. A novel colorimetric aptasensor using gold nanoparticle for a highly sensitive and specific detection of oxytetracycline. Biosens. Bioelectron. 2010;26:1644–1649. doi: 10.1016/j.bios.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 85.Xu Y., Lu C., Sun Y., Shao Y., Cai Y., Zhang Y., Miao J., Miao P. A colorimetric aptasensor for the antibiotics oxytetracycline and kanamycin based on the use of magnetic beads and gold nanoparticles. Microchim. Acta. 2018;185:548. doi: 10.1007/s00604-018-3077-y. [DOI] [PubMed] [Google Scholar]
- 86.He L., Luo Y., Zhi W., Wu Y., Zhou P. A Colorimetric Aptamer Biosensor Based on Gold Nanoparticles for the Ultrasensitive and Specific Detection of Tetracycline in Milk. Aust. J. Chem. 2013;66:485–490. doi: 10.1071/CH12446. [DOI] [Google Scholar]
- 87.Ramezani M., Danesh N.M., Lavaee P., Abnous K., Taghdisi S.M. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline. Biosens. Bioelectron. 2015;70:181–187. doi: 10.1016/j.bios.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 88.Luo Y., Xu J., Li Y., Gao H., Guo J., Shen F., Sun C. A novel colorimetric aptasensor using cysteamine-stabilized gold nanoparticles as probe for rapid and specific detection of tetracycline in raw milk. Food Control. 2015;54:7–15. doi: 10.1016/j.foodcont.2015.01.005. [DOI] [Google Scholar]
- 89.Sheng Y.-M., Liang J., Xie J. Indirect competitive determination of tetracycline residue in honey using an ultrasensitive gold-nanoparticle-linked aptamer assay. Molecules. 2020;25:2144. doi: 10.3390/molecules25092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen A., Jiang X., Zhang W., Chen G., Zhao Y., Tunio T.M., Liu J., Lv Z., Li C., Yang S. High sensitive rapid visual detection of sulfadimethoxine by label-freeaptasensor. Biosens. Bioelectron. 2013;42:419–425. doi: 10.1016/j.bios.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 91.Yan J., Huang Y., Fang Z., Bai W., Zhu C., Zhang C., Yan M., Chen A. Aptamer based photometric assay for the antibiotic sulfadimethoxine based on the inhibition and reactivation of the peroxidase-like activity of gold nanoparticles. Microchim. Acta. 2016;184:59–63. doi: 10.1007/s00604-016-1994-1. [DOI] [Google Scholar]
- 92.Chen X.-X., Lin Z.-Z., Hong C.-Y., Yao Q.-H., Huang Z.-Y. A dichromatic label-free aptasensor for sulfadimethoxine detection in fish and water based on AuNPs color and fluorescent dyeing of double-stranded DNA with SYBR green I. Food Chem. 2020;309:125712. doi: 10.1016/j.foodchem.2019.125712. [DOI] [PubMed] [Google Scholar]
- 93.Yu Z.-G., Lai R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of ampicillin in complex samples. Talanta. 2018;176:619–624. doi: 10.1016/j.talanta.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 94.Peng X., Tan J., Tang C., Yu Y., Wang Z. Multiresidue determination of fluoroquinolone, sulfonamide, trimethoprim, and chloramphenicol antibiotics in urban waters in China. Environ. Toxicol. Chem. 2008;27:73–79. doi: 10.1897/06-650.1. [DOI] [PubMed] [Google Scholar]
- 95.Popelka P., Nagy J., Marcincak S. A comparison of BSDA and PREMI test sensitivity to penicillin standards in poultry meat and after the administration of Amuril plv. sol. Folia Vet. 2003;47:139–141. [Google Scholar]
- 96.Jadhav R.W., Al Kobaisi M., Jones L.A., Vinu A., Bhosale S.V. The supramolecular self-assembly of aminoglycoside antibiotics and their applications. ChemistryOpen. 2019;8:1154–1166. doi: 10.1002/open.201900193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.González-Fernández E., De-Los-Santos-Álvarez N., Lobo-Castañón M.J., Miranda-Ordieres A.J., Tuñón-Blanco P. Impedimetric aptasensor for tobramycin detection in human serum. Biosens. Bioelectron. 2011;26:2354–2360. doi: 10.1016/j.bios.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 98.Haasnoot W., Cazemier G., Koets M., Van Amerongen A. Single biosensor immunoassay for the detection of five aminoglycosides in reconstituted skimmed milk. Anal. Chim. Acta. 2003;488:53–60. doi: 10.1016/S0003-2670(03)00628-7. [DOI] [Google Scholar]
- 99.Chen S.-H., Liang Y.-C., Chou Y.-W. Analysis of kanamycin A in human plasma and in oral dosage form by derivatization with 1-naphthyl isothiocyanate and high-performance liquid chromatography. J. Sep. Sci. 2006;29:607–612. doi: 10.1002/jssc.200500450. [DOI] [PubMed] [Google Scholar]
- 100.Granja R.H., Niño A.M.M., Zucchetti R.A., Niño R.E.M., Patel R., Salerno A.G. Determination of streptomycin residues in honey by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta. 2009;637:64–67. doi: 10.1016/j.aca.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 101.Yadavalli R.K., Singh R.S., Anand S.K. Diffusion test for the detection of streptomycin residues in milk. J. Dairy Res. 1985;52:595–597. doi: 10.1017/S0022029900024559. [DOI] [PubMed] [Google Scholar]
- 102.Lian W., Liu S., Yu J., Li J., Cui M., Xu W., Huang J. Electrochemical sensor using neomycin-imprinted film as recognition element based on chitosan-silver nanoparticles/graphene-multiwalled carbon nanotubes composites modified electrode. Biosens. Bioelectron. 2013;44:70–76. doi: 10.1016/j.bios.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Wang S., Xu B., Zhang Y., He J. Development of enzyme-linked immunosorbent assay (ELISA) for the detection of neomycin residues in pig muscle, chicken muscle, egg, fish, milk and kidney. Meat Sci. 2009;82:53–58. doi: 10.1016/j.meatsci.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 104.Wochner A., Menger M., Orgel D., Cech B., Rimmele M., Erdmann V.A., Glökler J. A DNA aptamer with high affinity and specificity for therapeutic anthracyclines. Anal. Biochem. 2008;373:34–42. doi: 10.1016/j.ab.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 105.Ferguson J., Baxter A., Young P., Kennedy G., Elliott C., Weigel S., Gatermann R., Ashwin H., Stead S., Sharman M. Detection of chloramphenicol and chloramphenicol glucuronide residues in poultry muscle, honey, prawn and milk using a surface plasmon resonance biosensor and Qflex® kit chloramphenicol. Anal. Chim. Acta. 2005;529:109–113. doi: 10.1016/j.aca.2004.11.042. [DOI] [Google Scholar]
- 106.Ding Y., Zhang X., Yin H., Meng Q., Zhao Y., Liu L., Wu Z., Xu H. Quantitative and sensitive detection of chloramphenicol by surface-enhanced raman scattering. Sensors. 2017;17:2962. doi: 10.3390/s17122962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miao Y., Gan N., Li T., Zhang H., Cao Y., Jiang Q. A colorimetric aptasensor for chloramphenicol in fish based on double-stranded DNA antibody labeled enzyme-linked polymer nanotracers for signal amplification. Sens. Actuators B Chem. 2015;220:679–687. doi: 10.1016/j.snb.2015.05.106. [DOI] [Google Scholar]
- 108.Zhang X.-H., Deng Y., Zhao M.-Z., Zhou Y.-L., Zhang X.-X. Highly-sensitive detection of eight typical fluoroquinolone antibiotics by capillary electrophoresis-mass spectroscopy coupled with immunoaffinity extraction. RSC Adv. 2018;8:4063–4071. doi: 10.1039/C7RA12557G. [DOI] [Google Scholar]
- 109.Scherer R., Pereira J., Firme J., Lemos M., Lemos M. Determination of ciprofloxacin in pharmaceutical formulations using HPLC method with UV detection. Indian J. Pharm. Sci. 2015;76:541–544. [PMC free article] [PubMed] [Google Scholar]
- 110.Speltini A., Sturini M., Maraschi F., Profumo A. Fluoroquinolone antibiotics in environmental waters: Sample preparation and determination. J. Sep. Sci. 2010;33:1115–1131. doi: 10.1002/jssc.200900753. [DOI] [PubMed] [Google Scholar]
- 111.Wang Z., Zhu Y., Ding S., He F., Beier R.C., Li J., Jiang H., Feng C., Wan Y., Zhang S., et al. Development of a monoclonal Antibody-Based Broad-Specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Anal. Chem. 2007;79:4471–4483. doi: 10.1021/ac070064t. [DOI] [PubMed] [Google Scholar]
- 112.Matsunaga T., Kondo T., Osasa T., Kotsugai A., Shitanda I., Hoshi Y., Itagaki M., Aikawa T., Tojo T., Yuasa M. Sensitive electrochemical detection of ciprofloxacin at screen-printed diamond electrodes. Carbon. 2020;159:247–254. doi: 10.1016/j.carbon.2019.12.051. [DOI] [Google Scholar]
- 113.Chen T., Liu Y., Lu J., Xing J., Li J., Liu T., Xue Q. Highly efficient detection of ciprofloxacin in water using a nitrogen-doped carbon electrode fabricated through plasma modification. N. J. Chem. 2019;43:15169–15176. doi: 10.1039/C9NJ03511G. [DOI] [Google Scholar]
- 114.Johnson A.C., Keller V., Dumont E., Sumpter J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total. Environ. 2015;511:747–755. doi: 10.1016/j.scitotenv.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 115.Granados-Chinchilla F., Rodríguez C. Tetracyclines in food and feedingstuffs: From regulation to analytical methods, bacterial resistance, and environmental and health implications. J. Anal. Methods Chem. 2017;2017:1–24. doi: 10.1155/2017/1315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jalalian S.H., Taghdisi S.M., Danesh N.M., Bakhtiari H., Lavaee P., Ramezani M., Abnous K. Sensitive and fast detection of tetracycline using an aptasensor. Anal. Methods. 2015;7:2523–2528. doi: 10.1039/C5AY00225G. [DOI] [Google Scholar]
- 117.Khosrokhavar R., Hosseini M.-J., Amini M., Pirali-Hamedani M., Ghazi-Khansari M., Bakhtiarian A. Validation of an analytical methodology for determination of oxytetracycline residue in milk by HPLC with UV detection. Toxicol. Mech. Methods. 2008;18:351–354. doi: 10.1080/15376510701610984. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y., Yang Q., Zhang D., Gan N., Li Q., Cuan J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018;262:137–143. doi: 10.1016/j.snb.2018.01.218. [DOI] [Google Scholar]
- 119.He L., Luo Y., Zhi W., Zhou P. Colorimetric sensing of tetracyclines in milk based on the assembly of cationic conjugated polymer-aggregated gold nanoparticles. Food Anal. Methods. 2013;6:1704–1711. doi: 10.1007/s12161-013-9577-9. [DOI] [Google Scholar]
- 120.Jamshaid T., Tenório-Neto E.T., Baraket A., Lebaz N., Elaissari A., Sanchís A., Salvador J.-P., Marco M.-P., Bausells J., Errachid A., et al. Development of novel magneto-biosensor for sulfapyridine detection. Biosensors. 2020;10:43. doi: 10.3390/bios10040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dai T., Duan J., Li X., Xu X., Shi H., Kang W. Determination of sulfonamide residues in food by capillary zone electrophoresis with on-line chemiluminescence detection based on an Ag(III) Complex. Int. J. Mol. Sci. 2017;18:1286. doi: 10.3390/ijms18061286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang J., Wang G. IOP Conference Series: Earth and Environmental Science. IOP Publishing; Singapore: 2017. Hazard of sulfonamides and detection technology research progress. [Google Scholar]
- 123.Mohammad-Razdari A., Ghasemi-Varnamkhasti M., Izadi Z., Rostami S., Ensafi A.A., Siadat M., Losson E. Detection of sulfadimethoxine in meat samples using a novel electrochemical biosensor as a rapid analysis method. J. Food Compos. Anal. 2019;82:103252. doi: 10.1016/j.jfca.2019.103252. [DOI] [Google Scholar]
- 124.Du J., Jiang Q., Lu X., Chen L., Zhang Y., Xiong X. Detection of sulfadimethoxine using optical images of liquid crystals. Analyst. 2019;144:1761–1767. doi: 10.1039/C8AN02049C. [DOI] [PubMed] [Google Scholar]