Figure 2.
Cell length varies throughout the fixation protocol
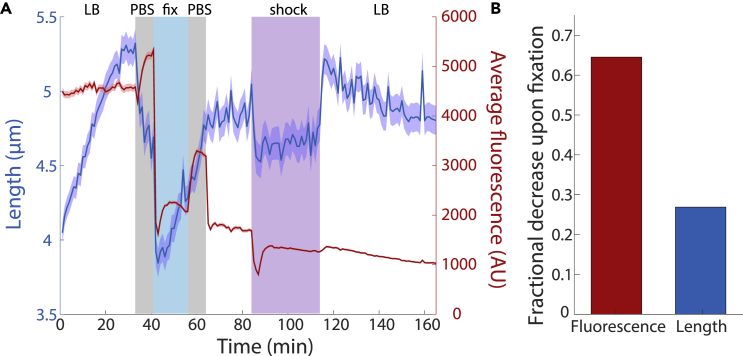
(A) Mean cell length (blue) and fluorescence (red) for E. coli MG1655 cells expressing cytoplasmic GFP tracked in a microfluidic flow cell (transparent methods) and subjected to the fixation protocol followed by osmotic shocks. Cells were equilibrated for 30 min in LB to achieve exponential growth and then switched to 1X PBS for 8 min to mimic washing. Cells were then fixed in PBS with 1X Chemicon for 15 min and transferred back to PBS for a second wash. To ensure successful fixation, cells were switched to LB for 20 min and no growth occurred. Finally, cells were exposed to a hyperosmotic shock with LB+1 M sorbitol for 30 min, which demonstrated that the membranes still acted as a permeability barrier (Figure S2A). Length varied over many steps of the protocol, while fluorescence decreased dramatically primarily upon fixation. The slow decrease after fixation is likely due to photobleaching. Lines are mean values, and shaded regions represent 1 standard error of the mean (n = 150 cells).
(B) The fractional decrease in cell length and fluorescence between t = 30 min (just before the first PBS wash) and 43 min (just after introduction of the fixative).