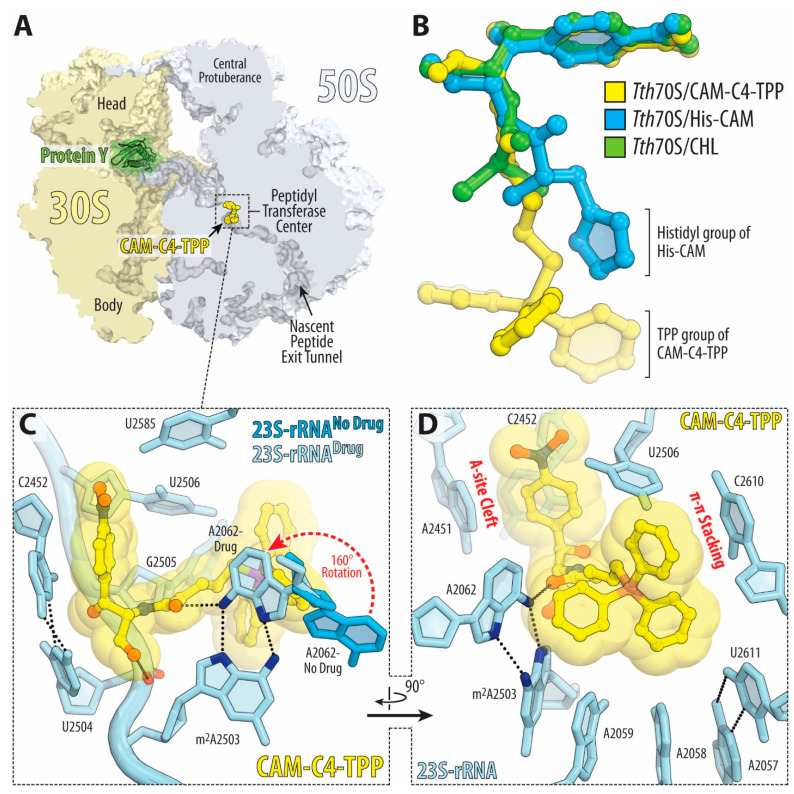
Figure 4.
Structure of CAM-C4-TPP in complex with the 70S ribosome. (A) Overview of the drug-binding site (yellow) in the T. thermophilus 70S ribosome viewed as a cross-cut section through the nascent peptide exit tunnel. The 30S subunit is shown in light yellow, the 50S subunit is in light blue, ribosome-bound protein Y is colored green. (B) Superposition of the ribosome-bound CAM-C4-TPP (yellow) with the previous structures of CHL (green, PDB entry 6ND5 [2]) or CHL analog histidyl-CAM (blue, PDB entry 6CFJ [13]). All structures were aligned based on domain V of the 23S rRNA. (C,D) Close-up views of the CHL analog CAM-C4-TPP bound in the PTC and NPET of the 70S ribosome (E. coli numbering of the 23S rRNA nucleotides is used). Potential H-bond interactions are indicated with dashed lines. Note that by forming an H-bond with the base of nucleotide A2062 of the 23S rRNA (light blue) CAM-C4-TPP causes characteristic rotation of this nucleotide by approximately 160 degrees to form Hoogsteen base-pair with the m2A2503 of the 23S rRNA (red dashed arrow). N6 and N7 atoms of nucleotides A2062 and m2A2503 are highlighted in dark blue. The unrotated conformation of A2062 observed in the absence of the drug is shown in blue (PDB entry 4Y4P [32]).