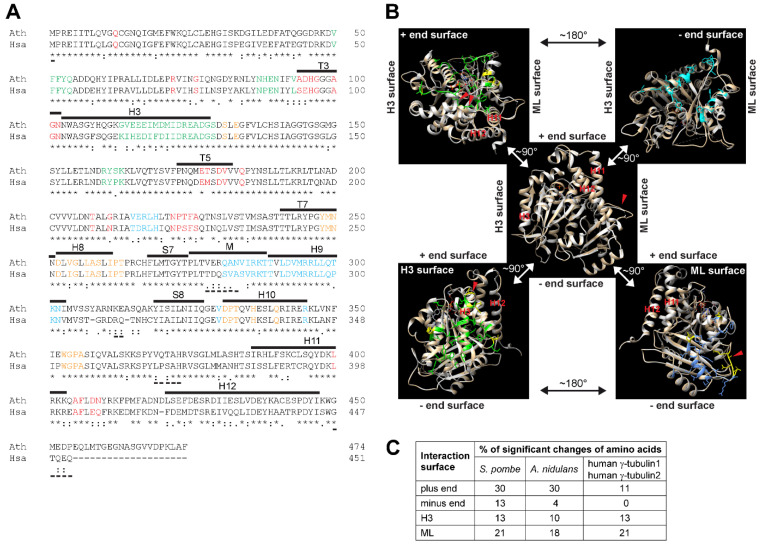
Figure 1.
(A) The protein sequence alignment of Arabidopsis and human γ-tubulins. Amino acids corresponding to those involved in longitudinal and lateral contacts in α- and β-tubulins are colored: red—plus end surface; orange—minus end surface; green—H3 surface; azure—ML surface. Secondary structures or loops are marked with lines above sequences according to human γ-tubulin PDB ID 3cb2a: H—helix; S—β-sheet; T/M—loop. Identical amino acids are marked under sequences according to ClustalW (T-Coffee) with an asterisk, conserved substitutions of the same size and hydropathy with colon, and semi-conserved substitutions of similar size or hydropathy with dot. Underscore under sequences marks amino acids not visible in PDB ID 3cb2a structure: Ath—γ-tubulin1 from Arabidopsis thaliana; has—γ-tubulin1 from Homo sapiens. (B) Comparison of protein structures of Arabidopsis and human γ-tubulin1. Cartoon representations of a protein structure model of Arabidopsis γ-tubulin1 obtained from a Swiss model (tan) aligned with PDB ID 3cb2a human γ-tubulin1 (white) using Chimera. Amino acids that differed significantly between Arabidopsis and human γ-tubulin1 are marked in yellow (semi-and non-conservative T-Coffee). In the center, there is a marked orientation of plus end and minus end surfaces and H3 and ML surfaces needed for longitudinal and lateral interactions, respectively; helices H11 and H12, H9-S8 loop (red arrowhead). Upper left corner—amino acids involved in longitudinal interactions at a plus end surface (green); changed amino acids are generally smaller and/or less polar than those of human γ-tubulin1; change of HWY motif (red arrowheads); upper right corner—amino acids involved in longitudinal interactions at minus end surface (cyan) includes no significantly different amino acids; bottom left corner—amino acids involved in lateral interactions on H3 surface (green); His forming a bulge in helix H3 (red arrowhead) is present in Arabidopsis γ-tubulin1, while it is absent in human γ-tubulin; bottom right corner—amino acids involved in lateral interactions on ML surface (cornflower blue); changed amino acids in Arabidopsis γ-tubulin1 are larger with only one exception (red arrowhead). GDP (orange stick). (A,B) Adapted with permission from ref. [3] Copyright 2021 Elsevier. (C) Sequence homology at interaction surfaces of γ-tubulin. Significant changes of amino acids (%) in Homo sapiens γ-tubulin1/2, Schizosaccharomyces pombe and Aspergillus nidulans γ-tubulin compared with γ-tubulin1 of Arabidopsis thaliana.