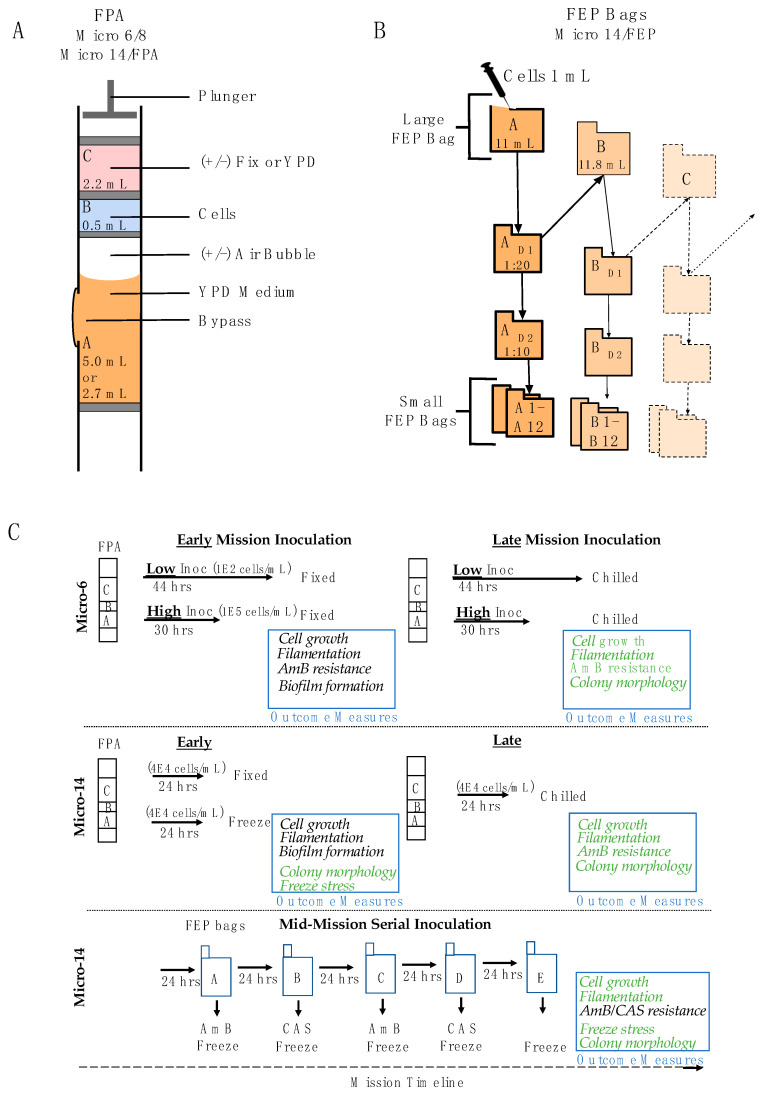
Figure 1.
Schematic representation of the hardware and experimental designs used in the mission payloads. (A) FPA bioreactors were used for the Micro-6/8 and Micro-14/FPA payloads and were loaded with two or three chambers, loaded in sequence, and separated by rubber septa. Medium was loaded in chamber A (2.7 mL in three chamber design, 5 mL in two chamber design) and cell inoculum was loaded in chamber B (0.5 mL). In the three chamber design, chamber C was loaded with 2.2 mL of fixative or fresh medium. Chambers were sequentially mixed on a predetermined schedule by depressing a plunger, and consequently the column of reagents and septa, to allow mixing of reagents through the bypass in the glass barrel. All eight FPAs within a GAP were processed as a unit. At the conclusion of the experimental operations, the FPAs were stored within the GAP at the prescribed temperature. (B) FEP bags were used for the Micro-14/FEP payload, allowing for dilution (D1 and D2 bags) and serial subculture procedures. To activate growth, yeast (1 mL, 4 × 104 cells/mL) was injected into the first Large FEP bag (Bag A containing 11 mL YPD) and cultured for 24 h +/− 1 h at 30 °C. At the end of this growth stage, an aliquot was removed and diluted 20-fold (Bag AD1). From this dilution, the next Large FEP bag was inoculated (0.25 mL into Bag B containing 11.8 mL YPD) and cultured for 24 h +/− 1 h at 30 °C. This process was repeated until the cells had been cultured for five days (through Bag E). On each of the first four days, samples were also prepared to analyze antifungal sensitivity. For these samples, the dilution series was extended from the D1 dilution bag to the D2 dilution bag (1:10) and aliquots (0.2 mL) from D2 were used to inoculate Small FEP bags (A1–A12, B1–B12, etc.) containing antifungal agents or control medium (2.8 mL). Each day following the 24 h growth period and any necessary sampling, FEP bags (Large FEP bags A–E, dilution D1 bags A–D, and Small FEP antifungal bags) were frozen at <−80 °C for the remainder of the mission. (C) An overview is provided to summarize the experimental details for each payload. Cell inoculation, time of cultivation, relative time within the mission, and storage conditions are provided. In addition, the outcome measurements employed for each payload are indicated. Outcome measures that were performed/completed during flight with cells fixed in microgravity are indicated in black text. Outcome measures that were performed using cells returned viable (chilled or frozen) and processed post flight in the home laboratory are indicated in green text.