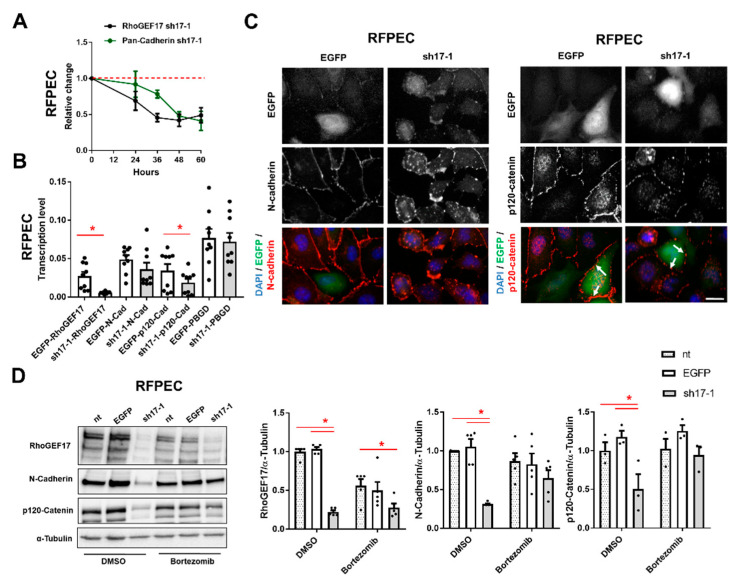
Figure 2.
Loss of RhoGEF17 results in the proteasomal degradation of AJ proteins in RFPEC. RFPEC were transduced with adenoviruses encoding EGFP (EGFP), or EGFP and a shRNA against RhoGEF17 (sh17-1). (A) Immunoblot analysis of RhoGEF17, pan-cadherin and α-tubulin was performed at the indicated time points. The values were normalized by α-tubulin and are given as means ± SEM relative to the time point 0 h, n = 3–21. (B) Immunofluorescence analysis was performed 48 h after transduction. Depicted are EGFP images, immunofluorescence staining of N-cadherin (left), p120-catenin (right) and the overlays with DAPI. Scale bar = 20 µm. (C) The transcript levels of RhoGEF17, N-cadherin, p120-catenin and the housekeeping gene PBGD were determined by qPCR after 48 h of transduction. The values are given as means + SEM with the single data points, n = 9, * p < 0.05 assessed by paired t-testing. (D) The proteasome was inhibited with 100 nM Bortezomib for 2 h. Non-transduced (nt) cells were used as additional control. RhoGEF17, N-cadherin and p120-catenin were detected by immunoblot in whole cell lysates. Shown are representative immunoblots of RhoGEF17, N-cadherin, p120-catenin and α-tubulin (left) and the quantitative analyses. Values were normalized by α-tubulin and are given relative to non-transduced cells treated with DMSO only. Shown are means + SEM and the single data points; n = 3–5, * p < 0.05 assessed by 2-way ANOVA with Tukey’s multiple comparison testing.