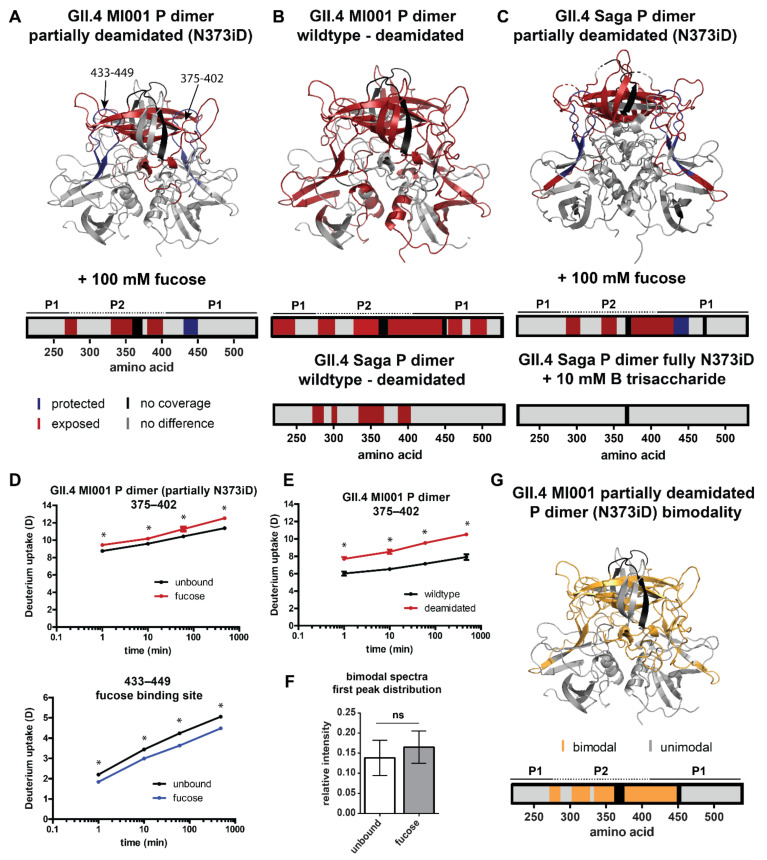
Figure 4.
Significant HDX differences in partially deamidated (N373iD) GII.4 P dimers. A/C/D) Fucose can still bind to the canonical glycan binding site in partially deamidated GII.4 MI001 (A) and GII.4 Saga (C) P dimers (pdb 6H9V). In contrast to the wildtype N373 P dimer, parts of the P2 domain get more exposed upon interaction with 100 mM fucose. HDX differences in fully deamidated GII.4 Saga P dimers in presence of 10 mM HBGA B trisaccharide are shown for comparison [15]. (B,E) The partially deamidated GII.4 MI001 N373iD P dimer shows a higher deuterium uptake in large parts of the structure, which points towards higher flexibility, like in the fully deamidated GII.4 Saga P dimer shown for comparison [15]. (D,E) Deuterium uptake plots exemplarily highlight statistically significant (*) deuteration differences in partially deamidated GII.4 MI001 P dimers in presence of fucose (D) and in comparison to the wildtype protein (E). All uptake plots show deuteration values of the second (main) peak distribution in case of bimodality. (F,G) Bimodality occurs in similar regions as for the wildtype P dimer, implying that the more protected subpopulation is also present in the partially deamidated sample (G). In contrast to the native P dimer, the relative intensity of the first peak distribution does not significantly (ns) increase under fucose treatment as shown in the bar plot in panel (F) (for statistics refer to description of Figure 3). Error bars represent the standard deviation.