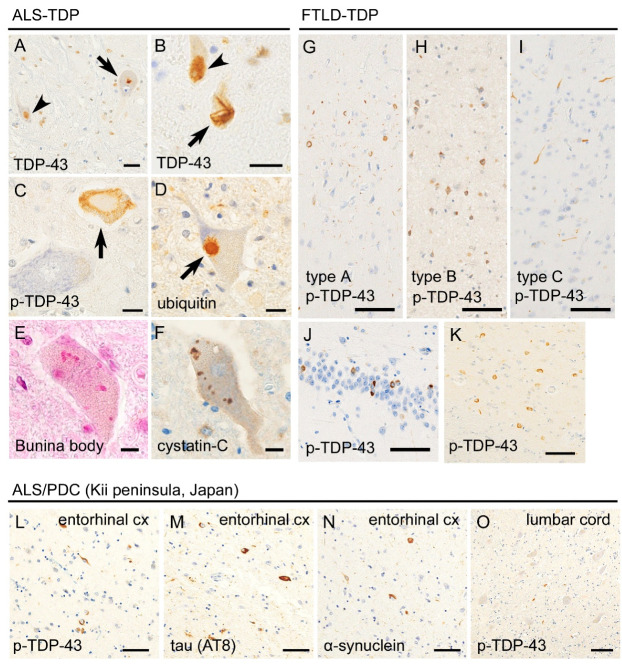
Figure 2.
Histopathological findings of transactivation response DNA binding protein 43kDa (TDP-43)-related ALS (ALS-TDP) and FTLD (FTLD-TDP). Panels A–D were taken from an ALS-TDP patient. Anti-TDP-43 immunohistochemistry revealed that TDP-43 was mislocalized from the nucleus to the cytoplasm and forms dot-like (A, arrow) or skein-like inclusions (B, arrow) in the spinal motor neuron. Unaffected neurons showed nuclear localization of TDP-43 (A and B, arrowheads). Anti-phosphorylated TDP-43 (p-TDP-43) immunohistochemistry revealed pathologic inclusions (C, arrow) but not the normal nuclear expression of TDP-43. TDP-43 inclusions were immunopositive for ubiquitin (D, arrow). Bunina bodies are also observed in the motor neurons of ALS-TDP patients (E) and immunolabeled with cystation-C (F). Panels G–K were taken from FTLD-TDP patients. Sporadic FTLD-TDP is classified into types A, B, and C; type A is characterized by short dystrophic neurites and round- or crescent-shaped neuronal inclusions in the superficial layers of the cerebral cortex; type B is characterized by ring-shaped neuronal inclusions across all cortical layers; and type C is characterized by long and thick immunopositivity of neurites in the superficial cortical layers (G–I). Hippocampal granule cells (J) and neostriatum (K) are also preferentially involved. Panels (L–O) were taken from an ALS/ parkinsonism-dementia complex (PDC) (Kii peninsula) patient. The entorhinal cortex (L–N) showed multiple proteinopathies, including p-TDP-43 (L), hyperphosphorylated tau (M), and α-synuclein (O). Relatively mild aggregation of p-TDP-43 was observed in the spinal cord, compared with classical ALS. Scale bars: (A,B) 20 μm, (C–F) 10 μm, and (G–O) 50 μm.